A Recent Study Compared The Vaccination anaphylaxis rates and revealed that COVID-19 vaccines rank fifth, behind rabies, tick-borne encephalitis, measles-mumps-rubella-varicella, and human papillomavirus vaccines. COMPARE.EDU.VN offers comprehensive analyses, empowering informed choices with balanced comparisons. Explore our resources for clarity on vaccination options, immunization schedules, and allergy considerations, ensuring you make educated decisions with our assistance for your well-being and the health of your loved ones.
1. What Did the Recent Study Reveal About Vaccination Anaphylaxis Rates?
The recent study revealed that COVID-19 vaccines are within the range of anaphylaxis rates reported across several common vaccines. The study, analyzing data from the US Vaccine Adverse Event Reporting System (VAERS) and the European EudraVigilance, compared anaphylaxis cases post-COVID-19 vaccination with those of other vaccines. This provides crucial context for understanding the safety profile of COVID-19 vaccines relative to other immunizations.
1.1. How Was the Data Collected and Analyzed?
The data was collected from VAERS and EudraVigilance, which are systems that monitor adverse reactions to vaccines. Researchers analyzed anaphylaxis cases reported from week 52/2020 through week 31/2021. The total number of COVID-19 vaccine doses administered during this period was also tracked to calculate anaphylaxis rates per million doses.
1.2. What Specific Vaccines Were Included in the Comparison?
The comparison included several common vaccines such as those for rabies, tick-borne encephalitis, measles-mumps-rubella-varicella (MMRV), and human papillomavirus (HPV). These vaccines were chosen because they have established anaphylaxis rates, providing a benchmark for evaluating the rates associated with COVID-19 vaccines.
1.3. What Were the Anaphylaxis Rates for COVID-19 Vaccines?
The mean anaphylaxis rate for COVID-19 vaccines was estimated at 10.67 cases per 1 million doses. This rate varied slightly depending on the specific vaccine, ranging from 7.99 to 19.39 cases per million doses. These figures are important for contextualizing the risk associated with COVID-19 vaccines.
1.4. How Did COVID-19 Vaccines Rank Compared to Other Vaccines?
COVID-19 vaccines ranked fifth in reported anaphylaxis rates, behind rabies, tick-borne encephalitis, MMRV, and HPV vaccines. This ranking helps to reassure the public that the risk of anaphylaxis from COVID-19 vaccines is comparable to or lower than that of other commonly administered vaccines.
1.5. What Factors Might Influence Anaphylaxis Rates?
Several factors can influence anaphylaxis rates, including the vaccine formulation, the individual’s immune response, and pre-existing allergies. The study also noted that increased awareness among healthcare professionals may lead to more frequent reporting of anaphylaxis cases.
2. Why Is It Important to Compare Vaccination Anaphylaxis Rates?
Comparing vaccination anaphylaxis rates is important for several reasons, including informing public health policy, increasing vaccine confidence, and improving risk communication. This data helps healthcare professionals and the public make informed decisions about vaccination.
2.1. How Does This Data Inform Public Health Policy?
Public health officials use anaphylaxis rate data to assess the safety profile of vaccines and to develop guidelines for vaccine administration. This data can also inform decisions about vaccine recommendations and prioritization.
2.2. How Does It Increase Vaccine Confidence?
By providing clear and transparent data on anaphylaxis rates, public health officials can increase public confidence in vaccines. Knowing that the risk of anaphylaxis is low and comparable to other vaccines can reassure individuals who may be hesitant to get vaccinated.
2.3. How Does It Improve Risk Communication?
Understanding the relative risk of anaphylaxis from different vaccines allows healthcare providers to communicate more effectively with patients. This helps patients make informed decisions about vaccination based on a clear understanding of the risks and benefits.
2.4. What Role Do Reporting Systems Like VAERS and EudraVigilance Play?
VAERS and EudraVigilance are crucial for monitoring vaccine safety. These systems allow healthcare professionals and the public to report adverse events following vaccination, providing valuable data for ongoing safety assessments.
2.5. How Can Healthcare Professionals Use This Information?
Healthcare professionals can use this information to counsel patients about the risks and benefits of vaccination. They can also use it to prepare for and manage potential anaphylactic reactions in clinical settings.
3. What Are the Key Findings of the Anaphylaxis Rate Study?
The key findings of the anaphylaxis rate study include the estimated mean anaphylaxis rate of 10.67 cases per million doses for COVID-19 vaccines, the ranking of COVID-19 vaccines fifth among those studied, and the identification of factors that may influence anaphylaxis rates.
3.1. What Was the Sample Size and Study Duration?
The study analyzed data from over 837 million COVID-19 vaccine doses administered in the US and Europe between week 52/2020 and week 31/2021. This large sample size provides robust data for assessing anaphylaxis rates.
3.2. What Were the Anaphylaxis Rates for Different COVID-19 Vaccines?
The anaphylaxis rates varied slightly among different COVID-19 vaccines:
- Spikevax (Moderna): 8.58 cases per million doses
- Comirnaty (Pfizer-BioNTech): 10.44 cases per million doses
- Vaxzevria (AstraZeneca): 19.39 cases per million doses
- Janssen (Johnson & Johnson): 7.99 cases per million doses
3.3. What Were the Fatal Anaphylaxis Cases Reported?
The study reported 52 fatal anaphylaxis cases out of over 837 million doses, corresponding to 0.06 fatal cases per million doses. This indicates that fatal anaphylaxis is an extremely rare event following COVID-19 vaccination.
3.4. How Does This Study Compare to Previous Research?
This study aligns with previous research indicating that anaphylaxis following COVID-19 vaccination is rare. It provides additional data and context by comparing anaphylaxis rates with other commonly administered vaccines.
3.5. What Are the Limitations of the Study?
The study’s limitations include the reliance on passive reporting systems, which may be subject to reporting bias. Additionally, the study cannot establish a definitive cause-and-effect relationship between vaccination and anaphylaxis.
4. How Do Anaphylaxis Rates Vary Among Different Vaccines?
Anaphylaxis rates vary among different vaccines due to differences in vaccine formulation, individual immune responses, and other factors. Understanding these variations is important for informed decision-making.
4.1. What Factors Contribute to Different Anaphylaxis Rates?
Factors that contribute to different anaphylaxis rates include:
- Vaccine formulation: Different vaccines contain different ingredients, some of which may be more likely to trigger allergic reactions.
- Individual immune response: Individuals may have different sensitivities and allergic predispositions.
- Administration route: The route of vaccine administration (e.g., intramuscular, subcutaneous) may affect the risk of anaphylaxis.
- Age and health status: Age and underlying health conditions can influence the immune response to vaccines.
4.2. How Do Routine Childhood Vaccines Compare?
Routine childhood vaccines generally have low anaphylaxis rates. For example, the MMR vaccine has an anaphylaxis rate of approximately 1 in 1 million doses. These low rates contribute to the overall safety profile of childhood vaccination programs.
4.3. What Are the Anaphylaxis Rates for Travel Vaccines?
Travel vaccines, such as those for yellow fever and Japanese encephalitis, may have higher anaphylaxis rates than routine vaccines. This is due to the different formulations and the populations to whom these vaccines are administered.
4.4. How Do mRNA Vaccines Compare to Traditional Vaccines?
mRNA vaccines, such as those developed by Pfizer-BioNTech and Moderna, have shown similar anaphylaxis rates to traditional vaccines. The study found that the anaphylaxis rates for these vaccines were within the range of other commonly administered vaccines.
4.5. What Precautions Can Be Taken to Minimize Anaphylaxis Risk?
Precautions to minimize anaphylaxis risk include:
- Screening for allergies: Healthcare providers should screen patients for known allergies before administering vaccines.
- Having emergency equipment available: Vaccination sites should be equipped with epinephrine and trained personnel to manage anaphylaxis.
- Monitoring patients after vaccination: Patients should be monitored for at least 15 minutes after vaccination to detect any signs of anaphylaxis.
5. What Is Anaphylaxis and How Is It Treated?
Anaphylaxis is a severe, life-threatening allergic reaction that can occur after vaccination. Understanding the symptoms and treatment of anaphylaxis is crucial for managing this potential adverse event.
5.1. What Are the Symptoms of Anaphylaxis?
Symptoms of anaphylaxis can include:
- Skin reactions: Hives, itching, flushing
- Respiratory symptoms: Wheezing, shortness of breath, throat swelling
- Cardiovascular symptoms: Rapid heartbeat, dizziness, loss of consciousness
- Gastrointestinal symptoms: Nausea, vomiting, diarrhea
5.2. How Quickly Do Symptoms Appear?
Symptoms of anaphylaxis typically appear within minutes of exposure to the allergen. In some cases, symptoms may be delayed for up to an hour.
5.3. What Is the Treatment for Anaphylaxis?
The primary treatment for anaphylaxis is an injection of epinephrine. Epinephrine helps to reverse the symptoms of anaphylaxis by opening the airways, increasing blood pressure, and reducing swelling.
5.4. What Should You Do If You Experience Anaphylaxis After Vaccination?
If you experience symptoms of anaphylaxis after vaccination, seek immediate medical attention. Use an epinephrine auto-injector if available, and call emergency services.
5.5. How Can Anaphylaxis Be Prevented?
Anaphylaxis can be prevented by:
- Identifying and avoiding known allergens: Individuals with known allergies should avoid exposure to those allergens.
- Informing healthcare providers of allergies: Patients should inform their healthcare providers of any allergies before receiving vaccines or medications.
- Having an emergency action plan: Individuals at risk of anaphylaxis should have an emergency action plan and carry an epinephrine auto-injector.
6. How Can Individuals With Allergies Safely Get Vaccinated?
Individuals with allergies can safely get vaccinated by taking certain precautions and working closely with their healthcare providers.
6.1. What Precautions Should Allergic Individuals Take?
Allergic individuals should:
- Consult with an allergist: An allergist can help assess the risk of allergic reactions to vaccines.
- Get vaccinated in a medical setting: Vaccination should occur in a setting where anaphylaxis can be promptly treated.
- Be monitored after vaccination: Patients should be monitored for at least 30 minutes after vaccination.
6.2. Can Skin Testing Help Determine Vaccine Allergies?
Skin testing may help determine whether an individual is allergic to a specific vaccine component. However, skin testing is not always accurate and should be interpreted by an allergist.
6.3. What Is the Role of an Allergist in Vaccine Decisions?
An allergist can help assess the risk of allergic reactions to vaccines, perform skin testing if necessary, and provide guidance on safe vaccination strategies.
6.4. How Can Desensitization Be Used for Vaccine Allergies?
Desensitization involves gradually exposing an individual to a vaccine component to reduce the risk of an allergic reaction. This approach may be used in cases where vaccination is essential and the risk of anaphylaxis is high.
6.5. What Are the Recommendations for Individuals With a History of Anaphylaxis?
Individuals with a history of anaphylaxis should consult with an allergist before getting vaccinated. Vaccination may be possible in a medical setting with appropriate monitoring and emergency equipment.
7. What Are the Public Perceptions of Vaccine Safety?
Public perceptions of vaccine safety are influenced by various factors, including media coverage, personal experiences, and trust in healthcare providers. Understanding these perceptions is important for addressing vaccine hesitancy.
7.1. How Does Media Coverage Affect Vaccine Perceptions?
Media coverage can significantly impact public perceptions of vaccine safety. Sensationalized or inaccurate reporting can lead to increased vaccine hesitancy.
7.2. What Role Do Social Media Play in Spreading Vaccine Misinformation?
Social media platforms can be a breeding ground for vaccine misinformation. False or misleading information can spread rapidly, eroding public trust in vaccines.
7.3. How Can Healthcare Providers Address Vaccine Hesitancy?
Healthcare providers can address vaccine hesitancy by:
- Providing accurate information: Healthcare providers should provide patients with evidence-based information about vaccine safety and efficacy.
- Addressing concerns: Healthcare providers should listen to and address patients’ concerns about vaccines.
- Building trust: Healthcare providers should build trusting relationships with their patients to foster open communication about vaccination.
7.4. What Strategies Can Improve Vaccine Confidence?
Strategies to improve vaccine confidence include:
- Public education campaigns: Public education campaigns can disseminate accurate information about vaccines and address common misconceptions.
- Community engagement: Engaging with communities can help build trust in vaccines and address local concerns.
- Transparency: Transparent communication about vaccine development, safety, and efficacy can increase public confidence.
7.5. How Can Reporting Systems Like VAERS Help Build Trust?
Reporting systems like VAERS can help build trust by demonstrating that vaccine safety is being actively monitored. These systems provide transparency and allow for the detection of potential safety issues.
8. What Research Is Being Done to Improve Vaccine Safety?
Ongoing research is focused on improving vaccine safety by developing new vaccine formulations, identifying risk factors for adverse events, and improving monitoring systems.
8.1. What Are the Latest Advances in Vaccine Technology?
Latest advances in vaccine technology include:
- mRNA vaccines: mRNA vaccines offer a rapid and flexible platform for vaccine development.
- Viral vector vaccines: Viral vector vaccines use a harmless virus to deliver genetic material into cells.
- Subunit vaccines: Subunit vaccines contain specific components of a pathogen to stimulate an immune response.
8.2. How Are Researchers Identifying Risk Factors for Adverse Events?
Researchers are using various methods to identify risk factors for adverse events, including:
- Epidemiological studies: Epidemiological studies can identify associations between certain factors and adverse events.
- Genetic studies: Genetic studies can identify genetic predispositions to adverse events.
- Immunological studies: Immunological studies can help understand the immune mechanisms underlying adverse events.
8.3. What Efforts Are Being Made to Improve Vaccine Monitoring Systems?
Efforts to improve vaccine monitoring systems include:
- Enhancing data collection: Improving the completeness and accuracy of adverse event reporting.
- Developing automated data analysis tools: Using machine learning and artificial intelligence to detect potential safety signals.
- Improving communication: Enhancing communication between healthcare providers, public health officials, and the public.
8.4. How Can Personalized Medicine Enhance Vaccine Safety?
Personalized medicine approaches can enhance vaccine safety by tailoring vaccination strategies to individual risk factors and immune responses.
8.5. What Role Do International Collaborations Play in Vaccine Safety Research?
International collaborations play a crucial role in vaccine safety research by sharing data, expertise, and resources. These collaborations can help identify and address global vaccine safety issues.
9. What Are the Long-Term Effects of COVID-19 Vaccines?
Long-term studies are ongoing to assess the long-term effects of COVID-19 vaccines. Current data indicate that the benefits of vaccination far outweigh the risks.
9.1. What Studies Are Tracking Long-Term Vaccine Effects?
Several studies are tracking long-term vaccine effects, including:
- Large-scale observational studies: These studies monitor vaccinated populations over extended periods.
- Clinical trials: Clinical trials continue to follow participants to assess long-term outcomes.
- Registry data: Vaccine registries provide data on vaccinated individuals over time.
9.2. What Are the Current Findings on Long-Term Safety?
Current findings on long-term safety indicate that COVID-19 vaccines are generally safe and effective. Serious adverse events are rare.
9.3. How Do Vaccines Affect Different Age Groups?
Vaccines may have different effects on different age groups. Studies are ongoing to assess the safety and efficacy of vaccines in children, adolescents, adults, and older adults.
9.4. What Is the Impact of Boosters on Long-Term Immunity?
Boosters can enhance long-term immunity by increasing antibody levels and strengthening the immune response. Studies are ongoing to determine the optimal timing and frequency of booster doses.
9.5. How Can Individuals Stay Informed About Vaccine Updates?
Individuals can stay informed about vaccine updates by:
- Consulting with healthcare providers: Healthcare providers can provide the latest information on vaccines.
- Following reputable sources: Following reputable sources such as the CDC, WHO, and academic journals.
- Staying informed about public health recommendations: Staying informed about public health recommendations regarding vaccination.
10. How Can COMPARE.EDU.VN Help You Make Informed Decisions About Vaccinations?
COMPARE.EDU.VN provides comprehensive and objective comparisons of different vaccines, helping you make informed decisions based on the latest scientific evidence.
10.1. What Resources Does COMPARE.EDU.VN Offer?
COMPARE.EDU.VN offers a variety of resources, including:
- Vaccine comparisons: Detailed comparisons of different vaccines, including their efficacy, safety, and side effects.
- Expert reviews: Reviews from healthcare professionals and experts in the field.
- User ratings: Ratings and reviews from other users.
- Educational articles: Articles that provide information about vaccines, allergies, and public health.
10.2. How Does COMPARE.EDU.VN Ensure Objectivity?
COMPARE.EDU.VN ensures objectivity by:
- Using evidence-based information: Providing information based on the latest scientific evidence.
- Avoiding bias: Presenting information in a neutral and unbiased manner.
- Consulting with experts: Consulting with healthcare professionals and experts in the field.
10.3. How Can You Use COMPARE.EDU.VN to Compare Different Vaccines?
You can use COMPARE.EDU.VN to compare different vaccines by:
- Searching for specific vaccines: Searching for the vaccines you are interested in.
- Comparing features: Comparing the features of different vaccines side-by-side.
- Reading reviews: Reading reviews from experts and other users.
10.4. What Information Is Available on Vaccine Side Effects?
COMPARE.EDU.VN provides detailed information on vaccine side effects, including common side effects, rare side effects, and how to manage them.
10.5. How Can COMPARE.EDU.VN Help You Address Vaccine Hesitancy?
COMPARE.EDU.VN can help you address vaccine hesitancy by providing accurate information, addressing concerns, and building trust in vaccines.
Making informed decisions about vaccinations is crucial for protecting your health and the health of your community. With the right information and resources, you can confidently choose the vaccines that are right for you.
For detailed comparisons and more information, visit COMPARE.EDU.VN, your trusted source for objective and comprehensive vaccine information. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or Whatsapp: +1 (626) 555-9090. Let compare.edu.vn help you make informed decisions about your health.
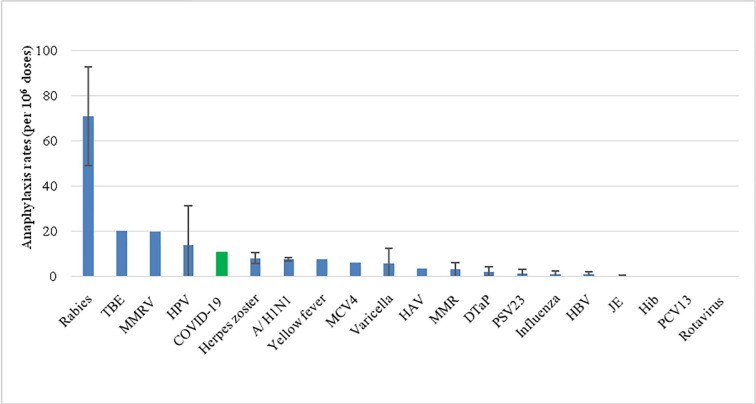 Comparison of anaphylaxis rates after vaccination
Comparison of anaphylaxis rates after vaccination
FAQ: Common Questions About Vaccination Anaphylaxis
1. What is the risk of anaphylaxis from vaccines?
The risk of anaphylaxis from vaccines is very low, typically ranging from 1 to 10 cases per million doses, depending on the vaccine.
2. Who is at higher risk of anaphylaxis from vaccines?
Individuals with a history of anaphylaxis to a previous vaccine or a component of the vaccine are at higher risk.
3. How soon do anaphylactic reactions typically occur after vaccination?
Anaphylactic reactions typically occur within minutes of vaccination, but can sometimes be delayed for up to an hour.
4. What are the common symptoms of anaphylaxis?
Common symptoms of anaphylaxis include hives, itching, flushing, wheezing, shortness of breath, and throat swelling.
5. How is anaphylaxis treated?
Anaphylaxis is treated with an injection of epinephrine, which helps to reverse the symptoms.
6. Can individuals with allergies safely get vaccinated?
Yes, individuals with allergies can safely get vaccinated by taking precautions and working with their healthcare providers.
7. What precautions should allergic individuals take before vaccination?
Allergic individuals should consult with an allergist, get vaccinated in a medical setting, and be monitored after vaccination.
8. Can skin testing help determine vaccine allergies?
Skin testing may help determine whether an individual is allergic to a specific vaccine component.
9. What should I do if I experience anaphylaxis after vaccination?
If you experience symptoms of anaphylaxis after vaccination, seek immediate medical attention.
10. How can I stay informed about vaccine safety updates?
Stay informed by consulting with healthcare providers, following reputable sources like the CDC and WHO, and staying updated on public health recommendations.