Introduction
The unprecedented spread of SARS-CoV-2 highlighted the critical need for intelligent and efficient diagnostic practices for COVID-19. The rapid propagation of the virus, coupled with limitations in reliable testing models, posed significant challenges for clinicians worldwide. While Artificial Intelligence (AI) has emerged as a powerful tool in medical image processing, significantly easing the burden of COVID-19 diagnosis, traditional AI approaches often rely on centralized data storage and training. This centralization introduces computational complexities and, more importantly, raises substantial privacy concerns, particularly in the healthcare sector where data sensitivity is paramount. The real-world scenario demands a global exchange of data across hospitals to develop robust predictive models, but this must be achieved while strictly adhering to organizational and patient privacy regulations. Therefore, collaborative model development and stringent privacy protection are essential considerations in training effective global deep learning models for disease diagnosis.
To address these challenges, this paper delves into a comparative analysis of a novel framework that synergistically integrates blockchain and the federated learning model. This integrated approach, known as FLED-Block (Federated Learning Ensembled Deep Five Learning Blockchain model), leverages the strengths of federated learning to mitigate computational complexity and blockchain to ensure distributed data management with robust privacy preservation. The FLED-Block framework facilitates data collection from diverse medical healthcare centers, fosters model development using a hybrid capsule learning network, and enables accurate COVID-19 prediction while maintaining data privacy and secure data sharing among authorized entities. Extensive experimental evaluations using lung CT images demonstrate the superior performance of the proposed model compared to established deep learning architectures such as VGG-16 and 19, AlexNets, ResNets-50 and 100, Inception V3, DenseNets-121, 119, and 150, MobileNets, and SegCaps. The FLED-Block framework achieved remarkable results in terms of accuracy (98.2%), precision (97.3%), recall (96.5%), specificity (33.5%), and F1-score (97%) in COVID-19 prediction, all while effectively safeguarding data privacy among heterogeneous users.
Keywords: compare blockchain federated learning model, image processing, artificial intelligence, extreme learning machine, privacy preservation, capsule learning model
Background and Motivation
The COVID-19 pandemic has underscored the devastating impact of infectious diseases on global health. The rapid spread of severe acute respiratory syndrome coronavirus (SARS-CoV-2) and its variants has placed immense strain on healthcare systems worldwide [1-3]. India, for example, has experienced a staggering number of confirmed cases and fatalities, highlighting the urgent need for effective diagnostic and management strategies [4, 5]. The sheer volume of infections overwhelmed healthcare providers, leading to challenges in timely and accurate diagnosis due to limitations in testing capacity and model reliability [6-8]. Diagnosis of COVID-19 typically involves a combination of clinical symptom assessment, epidemiological history, computed tomography (CT) scans, and pathogenic testing. However, CT scans, while valuable, often present similar visual patterns for COVID-19 and other lung disorders, complicating differential diagnosis [9-13]. Furthermore, the wide variability in COVID-19 symptom presentation and the disease’s rapid transmission rate further exacerbate diagnostic complexities [14].
This situation underscores the necessity for hospitals to collaboratively share patient data to improve diagnostic accuracy and develop more effective treatment strategies. However, secure data sharing, particularly sensitive patient information, and the development of global models for disease recognition present significant challenges. Current research paradigms often struggle to facilitate inter-hospital data exchange while upholding stringent privacy standards. AI-driven solutions, while promising, are often hampered by the fragmented nature of healthcare data and the lack of privacy-preserving methodologies for data sharing [15].
Existing AI techniques for medical image analysis frequently necessitate large datasets from single sources to effectively train deep learning models and achieve high prediction accuracy [16-23]. However, relying on single-source data can introduce feature distribution variance issues [24], potentially leading to higher misclassification rates and impacting diagnostic reliability. Data sharing across multiple hospitals could mitigate these limitations but is often restricted by legitimate security and privacy concerns. Therefore, there is a critical need to advance AI methodologies [25, 26] to facilitate collaborative learning paradigms that prioritize data privacy.
According to the World Health Organization (WHO), COVID-19 primarily affects the lungs, often resulting in characteristic honeycomb-like patterns. Even after recovery, some individuals experience long-term lung impairment. This highlights the importance of accurate and early detection of COVID-19-related lung pathologies to guide appropriate clinical interventions and minimize long-term complications. The motivation behind this research stems from the dual need to enhance COVID-19 diagnostic accuracy through AI and to address the critical challenges of data privacy in collaborative healthcare research [27]. Specifically, this study aims to develop a deep learning-based model for automated COVID-19 detection from lung CT scans, enabling data sharing for model training while preserving patient privacy.
The primary challenges addressed in this research are:
- Privacy Preservation: Overcoming the limitations imposed by privacy concerns that prevent the sharing of sensitive patient data necessary for training robust AI models.
- Federated Learning Integration: Utilizing a blockchain network to facilitate the training of a global diagnostic model through federated learning, enabling collaborative model development without centralized data aggregation.
- Data Scarcity and Model Improvement: Addressing the challenge of limited training data availability and enhancing prediction model accuracy to improve diagnostic precision.
- COVID-19 Pattern Recognition: Developing advanced AI techniques to accurately identify subtle and complex patterns indicative of COVID-19 infection in lung CT scans.
To overcome these challenges, the FLED-Block framework is proposed, designed to enable collaborative deep learning for COVID-19 diagnosis [28, 29] while ensuring robust privacy and security for participating healthcare institutions [30]. FLED-Block facilitates joint learning from diverse CT image datasets acquired from multiple sources. A novel capsule-deep extreme learning network is introduced within the framework to enhance image segmentation and classification accuracy. Capsule networks are particularly well-suited for medical image analysis due to their ability to effectively recognize subtle anomalies. To further improve model accuracy and diagnostic performance, the FLED-Block system combines the strengths of capsule networks and extreme learning machines (ELMs). ELMs replace traditional dense classification layers in capsule networks, enabling more efficient and accurate feature extraction and classification. To address privacy concerns, federated learning methodologies are employed to distribute the learned model across a decentralized network [31].
The key contributions of this research are:
- Novel Diagnostic Algorithm: Development of a novel algorithm for COVID-19 pattern detection and classification in CT images from multiple sources, utilizing an ensemble of capsule networks and extreme learning machines (ELMs) for enhanced feature extraction and classification accuracy.
- Blockchain-Powered Privacy-Preserving Data Sharing: Introduction of a blockchain-powered data collection and sharing mechanism that facilitates data aggregation from heterogeneous sources and employs federated learning to maintain data privacy while enabling high-accuracy global model training.
- Experimental Validation and Performance Superiority: Comprehensive experimental validation of the proposed algorithm using diverse datasets, demonstrating superior performance compared to existing deep learning algorithms in terms of key performance metrics.
This research introduces a blockchain-enabled federated framework to improve the recognition of COVID-19 from multi-source heterogeneous CT images and facilitate secure data sharing among hospitals while upholding patient privacy. The integration of ensembled capsule networks and ELMs enables effective feature extraction and classification, enhancing the accuracy of COVID-19 detection across diverse, publicly available CT image datasets.
The remainder of this paper is structured as follows: Section 2 reviews related works on decentralized networks in healthcare. Section 3 details the proposed FLED-Block model, including data normalization, the ensembled learning model, and the blockchain-based federated data sharing mechanism. Section 4 presents the experimental results, findings, and comparative analysis. Finally, Section 5 concludes the paper with a discussion of results, findings, and future research directions.
Related Works
Supriya et al. [24] investigated contemporary medical imaging analysis techniques for early disease detection, focusing on prediction, e-treatment, stage classification, virtual monitoring, and data transmission. Their study highlighted the use of supervised learning classifiers like SVM, DT, KNN, and ANN in medical imaging. They also emphasized the role of blockchain in facilitating secure and publicly accessible medical data transfer globally through distributed networks. The authors concluded that these innovations are transforming medical image transmission and healthcare delivery.
Recognizing the challenges in disseminating COVID-19 management information due to patient privacy concerns, researchers have explored privacy-preserving architectures. One such study proposed a federated learning and blockchain-based privacy architecture to enhance public communication and alternative information dissemination methods for COVID-19 [32]. This architecture aims to address data silos and enable shared model development while respecting data owner privacy and ensuring robust information security.
Georgios et al. [33] developed PriMIA, an open-source federated learning software framework for medical imaging, specifically for pediatric radiology classification across multiple data sources. PriMIA utilizes deep convolutional neural networks (DCNNs) to classify cardiac disease stages using pediatric chest X-ray images. The DCNN is trained with gradient-based models to detect chest diseases early. Shichang et al. [34] proposed a blockchain-based security model for federated learning to detect malicious nodes, combining competing vote authentication and aggregation strategies. Their model optimizes communication costs in federated learning with large datasets and mitigates “free-riding” and “model poisoning” attacks.
Kim et al. [35] introduced “BlockFL,” a blockchain federated learning architecture for decentralized federated learning, designed to overcome single points of failure by expanding the federated scope. BlockFL incorporates local training outcomes into a verification process to enhance trust in public networks. Bao et al. [36] presented FLchain, a centralized, publicly auditable federated learning ecosystem replacing the traditional central coordinator with blockchain to enhance trust and motivation in federated learning.
Majeed and Seon [37] proposed a channel-specific ledger-based blockchain architecture for federated learning, where global models are learned using channels. Local model parameters are stored as blocks in channel-specific ledgers, maintaining decentralized data management. Martinez et al. [38] explored cryptocurrency and federated learning to address data privacy and safety, proposing a detailed methodology with an off-chain record database for scalable gradient recording and reward mechanisms. Abdul Salam et al. [39] developed a federated learning algorithm for COVID-19 patients, pre-training a neural network with abnormal chest X-ray (CXR) images to predict mortality severity and guide e-treatment. However, their predictor was limited by data size.
Parnian et al. [40] introduced COVID-CAPS, a capsule network-based model to overcome CNN limitations with small datasets for COVID-19 detection from X-ray images. COVID-CAPS demonstrated superior performance compared to conventional networks when model parameters were optimized.
He et al. [41] conducted an experimental study on automated federated learning (AutoFL) using neural architecture search (NAS) and federated NAS (FedNAS) algorithms to improve the quality and efficiency of local machine learning models in federated settings. Their findings indicated that default parameters of local machine learning models might not be optimal for federated contexts, particularly for non-IID (non-independent and identically distributed) client data.
FLED-Block Model: Federated Learning and Blockchain Integration for COVID-19 Diagnosis
Hospitals and healthcare organizations are understandably hesitant to share patient data due to privacy concerns. However, effective deep learning models for disease diagnosis require substantial amounts of data. The FLED-Block model is proposed to address this data privacy paradox, enabling the training and sharing of global diagnostic models in a privacy-preserving manner. Figure 1 illustrates the architecture of the proposed FLED-Block framework.
Figure 1.
[Image of the proposed framework of the FLED-Block architecture, showcasing data flow from multiple hospitals through normalization, ensembled capsule network training, federated learning, and blockchain-based model sharing.]
Alt Text: FLED-Block framework architecture diagram illustrating data flow from diverse hospitals, encompassing data normalization, ensembled capsule network training, federated learning model aggregation, and secure blockchain-based model sharing for privacy-preserving COVID-19 diagnosis.
The FLED-Block architecture facilitates collaborative data collection from various hospitals, accommodating diverse CT scanner types. The initial step involves data normalization using spatial and signal normalization techniques to standardize the heterogeneous CT image data. Deep learning algorithms are then employed to identify COVID-19 patterns in lung CT images. Image segmentation and model training are performed using an ensembled capsule network to enhance generalization capability and diagnostic accuracy compared to traditional learning models.
The COVID-19 images are subsequently classified using extreme learning machines (ELMs). ELMs, known for their efficiency, are single hidden layer neural networks with self-tuning capabilities. Finally, federated learning is implemented to construct a global diagnostic model while addressing privacy concerns. This approach enables collaborative data utilization, adaptive model training, and model distribution across a public network, all while preserving data privacy through blockchain technology. Federated learning allows hospitals to maintain patient data privacy by exchanging only model weights and gradients via a secure blockchain network. This decentralized architecture ensures secure and private data communication across hospitals without compromising patient information security.
In essence, the FLED-Block framework operates in three key stages: (1) data collection from diverse medical healthcare centers, (2) model training using a hybrid capsule learning network for COVID-19 image segmentation and classification, and (3) collaborative sharing of the hybrid model using blockchain with federated learning, ensuring organizational privacy.
Materials and Methodologies
Artificial intelligence is increasingly essential in clinical diagnosis [42]. Deep learning algorithms, particularly, require large datasets for effective training, making data collection crucial for model validation [43]. This study utilized diverse heterogeneous CT image datasets for training and evaluating the proposed model. The datasets, designated as Dataset-1, Dataset-2, and Dataset-3, are described below.
Dataset-1
Dataset-1 comprises 34,006 CT scan slices from three hospitals, encompassing data from 89 individuals, with 28,395 slices from COVID-19-positive patients [44]. The data were acquired using six different CT scanners and include scans from 89 individuals, of whom 68 tested positive for COVID-19 and 21 tested negative. The distribution of collected CT image datasets is summarized in Table 1, and sample images are shown in Figure 2.
Figure 2.
[Example CT scan images from Dataset-1, illustrating variations in image characteristics.]
Alt Text: Representative Computed Tomography (CT) specimen images from Dataset-1, showcasing diverse lung scans utilized for COVID-19 diagnosis model training and evaluation.
Dataset-2
Dataset-2 consists of unenhanced chest CT scans from patients with confirmed COVID-19 infections [45]. Common comorbidities among these patients included hypertension, coronary heart disease, diabetes, interstitial pneumonia, and emphysema. Images were collected in inpatient settings from patients with positive Reverse Transcription Polymerase Chain Reaction (RT-PCR) tests for COVID-19 and associated clinical symptoms between March 2020 and January 2021. CT scans were performed in “Helical” mode using a NeuViz 16-slice CT scanner (Neu soft medical systems) without intravenous contrast. All images are in DICOM format, composed of 512 x 512 pixel 16-bit grayscale images.
Dataset-3
Dataset-3 includes 349 COVID-19 CT scans from 216 patients and 463 non-COVID-19 CT images. These datasets comprised initial images acquired at the point of care during an epidemic situation from patients with RT-PCR confirmation for SARS-CoV-2 presence. Detailed descriptions of Datasets-3 can be found in [46, 47].
To address the heterogeneity of data sources, a normalization approach was developed to adapt CT scan images for federated learning. Table 1 provides an overview of the datasets used to evaluate the proposed federated learning model. Figure 3 shows sample images from Datasets-2 and -3.
Table 1.
Summary of Dataset Details for Federated Learning Model Evaluation.
| Datasets | Source of datasets | No of COVID-19 patients | No of Non-COVID-19 infections | Image formats | Number of patients | Total number of CT scan images |
|---|---|---|---|---|---|---|
| Datasets-1 | CC-19 datasets [ ] | 63 | 26 | CT Scan images | 89 | 34,006 |
| Datasets-2 | COVID-19-CT datasets | 783 | 217 | DICOM Images | 1,000 | 45,002 |
| Dataset-3 | COVID-19 CT datasets [ ] | 216 | 463 | CT Scan Images/DICOM | 689 | 3,490 |
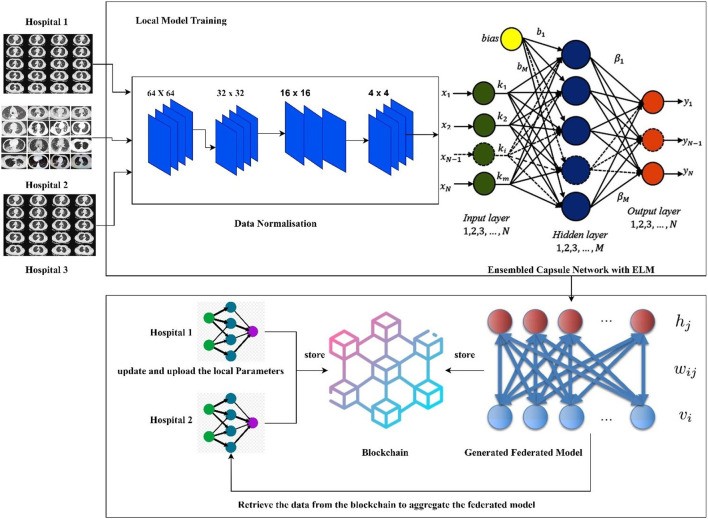
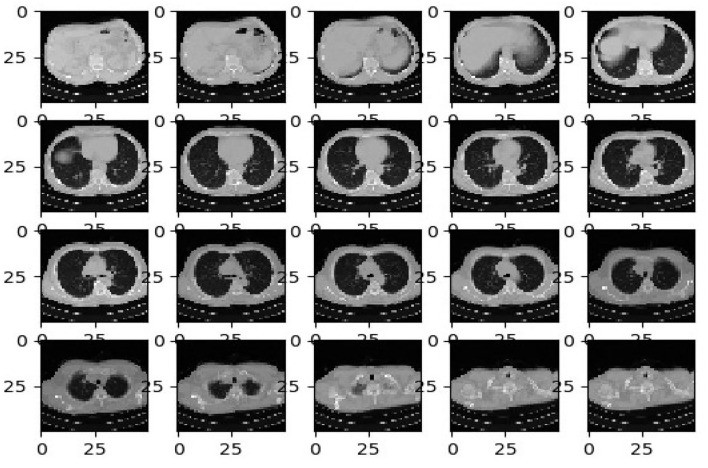
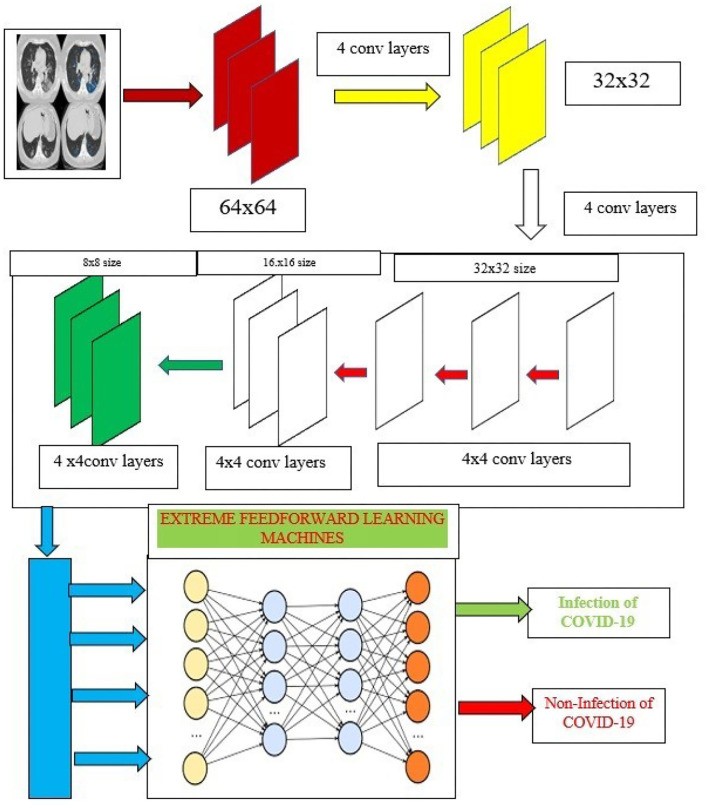
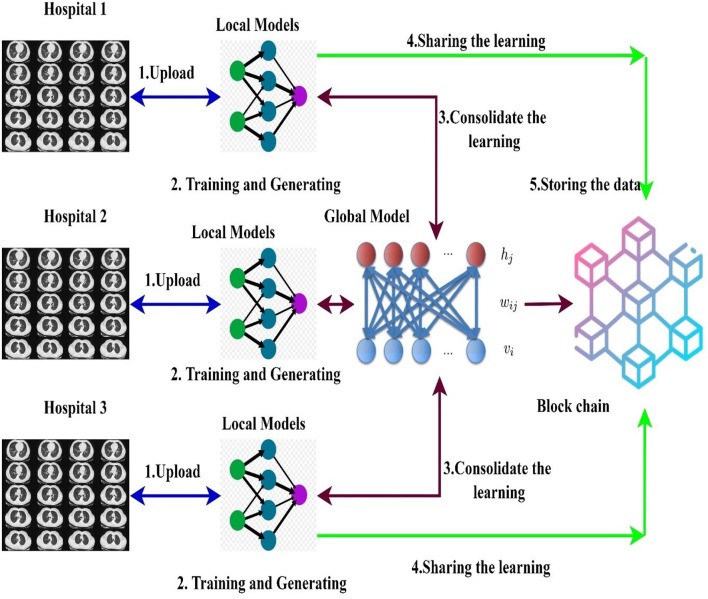
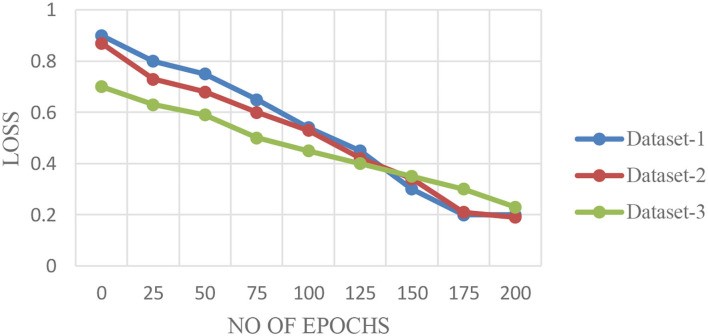
Figure 3.
[Example CT scan images from Datasets-2 and -3, showcasing different image characteristics and data sources.]
Alt Text: Representative Computed Tomography (CT) specimen images from Datasets-2 and Dataset-3, illustrating the diversity of image sources and formats used for training the federated learning model.
Data Normalization Techniques
The data normalization techniques described by Krizhevsky et al. [48] were adopted in this research. Given the use of heterogeneous data, robust normalization is essential to enhance the performance of federated learning models. Following Krizhevsky et al. [48], signal normalization and spatial normalization techniques were applied to process the CT scan images.
Signal Normalization Technique
Signal normalization adjusts voxel intensity based on the lung window. CT scans utilize Hounsfield Units (HU), with window level (WL) and window width (WW) being standard medical imaging parameters. Normalized values are calculated using Equation 1:
| $O_{normalized} = (O – WL) / WW$ | (1) |
|---|
Where $O_{normalized}$ represents the intensity-normalized image, and $O$ is the original input image. For this study, the lower bound window size was set within the range of [-0.05, 0.5].
Spatial Normalization Technique
Spatial normalization standardizes CT scan image dimensions and resolutions. All CT scan images were resampled to a standard resolution of 332 × 332 × 512 mm³, as suggested by Krizhevsky et al. [48]. This technique was applied to all datasets, normalizing all image formats to a standard format [49] suitable for federated learning, enhancing learning efficiency and model performance.
Ensembled Capsule-Based Model Training
Deep learning frameworks have gained significant prominence due to their powerful feature extraction and classification capabilities. Convolutional neural networks (CNNs) have been widely used for image classification. However, CNN pooling layers may not fully capture spatial relationships between image features, potentially increasing computational complexity and affecting classifier performance. To improve classification accuracy and diagnostic precision, the proposed system combines capsule networks and extreme learning machines (ELMs). Capsule networks are used for robust feature map extraction, while ELMs replace traditional dense classification layers for enhanced COVID-19 prediction.
Capsule Networks
Capsule networks [50] address CNN limitations using a hierarchical architecture comprising: (1) a convolutional layer, (2) a hidden layer, (3) a Primary Caps layer, and (4) a DigitCaps layer. Figure 4 shows the proposed training model architecture. Normalized input images are fed into the capsule network, which operates in two phases:
Figure 4.
[Diagram of the Capsule ensembled ELM layers, illustrating feature extraction and classification processes.]
Alt Text: Capsule ensembled Extreme Learning Machine (ELM) layer diagram detailing the architecture for feature extraction and classification in the proposed COVID-19 diagnostic model.
Capsule networks encode two critical properties:
- Probability of entity existence.
- Entity instantiation parameters.
Equation 2 encodes the spatial relationships between low-level and high-level features, using input vectors “s,” weight matrix “W,” and component vector “U.”
| $Y{(i.j)} = W{(i,j)}U_{(i,j)} * S_j$ | (2) |
|---|
Equation 3 calculates the current capsule “D” by summing weighted input vectors:
| $S{(j)} = sum{j} Y{(i,j)} * D{(j)}$ | (3) |
|---|
Finally, non-linearity is applied using the squash function (Equation 4):
| $Y{(i.j)} = W{i,j} U_{(i,j)} * S_j$ | (4) |
|---|
The distribution from low-level to high-level capsules is iteratively adjusted until an optimal distribution is achieved.
Extreme Learning Machines (ELMs)
The proposed research integrates capsule networks for superior feature extraction, enhancing classification accuracy. These extracted features are then input into extreme learning machines (ELMs) for image classification. ELMs are single hidden layer neural networks with autotuning properties, as shown in Figure 4.
ELMs offer performance advantages over other learning models like support vector machines (SVMs), Bayesian classifiers (BCs), K-nearest neighbors (KNN), and random forests (RFs) [51], particularly in terms of speed and reduced computational overhead. ELMs utilize kernel functions for high accuracy and performance. Key advantages include minimal training error and effective approximation, achieved through autotuning of weight biases and non-zero activation functions. Detailed ELM mechanisms are described in Huang et al. [52] and Wang et al. [53].
Mathematically, ELM characteristics are represented by:
| $fL(x) = sum{i=1}^{L} beta_i h_i(x) = h(x) beta$ | (5) |
|---|
where:
x → input
β → output weight vector:
| $beta = [beta_1, beta_2, ldots, beta_L]^T$ | (6) |
|---|
h(x) → output hidden layer:
| $h(x) = [h_1(x), h_2(x), ldots, h_L(x)]$ | (7) |
|---|
The ELM output is calculated by Equation 8:
| $f_L(x) = h(x) beta = h(x) H^T ( frac{1}{C} HH^T )^{-1} O$ | (8) |
|---|
where O represents output target vectors solved by hidden layer bias weights using Moore-Penrose pseudoinverse concepts [52]. Based on Equation 7, the infectious impact of COVID-19 on lungs is effectively classified. The operational mechanism of the proposed network is summarized in Algorithm 1.
Algorithm 1.
Pseudo code for the proposed ensembled algorithm for COVID-19 detection.
| 1 Inputs: Normalized Input Images: I |
|---|
| 2 Output: Presence of COVID-19 diseases on Lungs |
| 3 For n = 0 to Max_iterations n = Number of iterations |
| 4 Features F = Capsule(I) //Using Equations (2–4) |
| 5 Output Function = ELM(F) //Using Equation 7 |
| 6 If Output == threshold //User-defined threshold |
| 7 COVID-19 detected |
| 8 Else |
| 9 Normal Condition detected |
| 10 End If-Else |
| 11 End For Loop |
| 12 End Algorithm |
Federated Learning for Global Model Training
This section explores a decentralized data sharing mechanism involving multiple hospitals. The proposed model employs federated learning to enable hospital model sharing without compromising privacy, aggregating models from different hospitals. Consider H hospitals and a total dataset d. The ensembled learning model serves as the global model M, with ELM weights W randomly distributed to hospitals. Figure 5 illustrates the federated learning model used in this research.
Figure 5.
[Diagram of the Blockchain empowered federated learning model, illustrating data flow, local model training at hospitals, blockchain-based weight sharing, and global model aggregation.]
Alt Text: Blockchain empowered federated learning model diagram depicting data flow, local model training at individual hospitals, secure weight sharing via blockchain, and aggregation of local models to create a global COVID-19 diagnostic model.
A blockchain-based federated learning framework facilitates collaborative model training and sharing [50]. Hospitals utilize a global or collaborative model (“federated learning”) to integrate locally trained model weights [54]. Initially, data is collected from multiple sources into local models normalized to handle diverse CT scan data types. After normalization, images are segmented using ensembled capsule networks to train models for COVID-19 detection. Local model weights are then distributed across the blockchain network to train a global model.
Mathematically, let H be the number of hospitals, and d be the total dataset, comprising training and testing datasets as in Equation 9:
| $D{i}^{train} = {(X{i,j}^{train}, Y{i,j}^{train})} text{ where } j=1 text{ to } N{train} text{ data}$ | (9) |
|---|
Testing data is represented as in Equation 10:
| $D{i}^{test} = {(X{i,j}^{test}, Y{i,j}^{test})} text{ where } j=1 text{ to } N{test} text{ data}$ | (10) |
|---|
The total dataset for global model training is given by Equation 11:
| $D{(i)} = D{i}^{train} cup D_{i}^{test}$ | (11) |
|---|
Data D(i) from heterogeneous hospitals results in unequal data distribution. In each communication round, ELM weights W are distributed among hospitals. Hospitals create local models with received weights and store them on the blockchain. Weights are updated and uploaded to the blockchain in each round. Updated weights are mathematically expressed as in Equation 12:
| $eta = W_i – W_l$ | (12) |
|---|
where $W_i$ and $W_l$ are distributed global model weights and local model weights, respectively. All local models in the blockchain are aggregated to form a new learning model based on ELM principles, as implemented in the proposed deep learning algorithm.
Blockchain Framework for Federated Learning
The proposed framework incorporates blockchain architecture for secure and efficient data retrieval and sharing in federated learning. Multiple hospitals can collaboratively train models for improved disease detection. Based on multi-organization blockchain architectures [55, 56], the proposed approach outlines data retrieval and sharing processes.
Blockchain-Based Data Retrieval Process
Each hospital contributes data (local models) stored as blockchain transactions [57]. Data retrieval from nodes depends on node distance (d) and hospital ID. Hospital distance determines unique IDs, and the blockchain maintains logs of hospital IDs. Data is retrieved from neighboring hospitals identified by their unique IDs.
Mathematically, hospitals are denoted by X, partitioned into communities, with hospitals as nodes. Neighborhood distance between nodes is measured by Equation 13:
| $d(X{(i)}, X{(j)}) = frac{sum{p,q, in {X{(i)} cup X{(j)} – X{(i)} cap X{(j)}}} text{Attributes of Nodes}}{sum{p,q, in {X{(i)} cup X{(j)}}} text{Attributes of Nodes} * log(X{(i)}, X{(j)})}$ | (13) |
|---|
where $X{(i)}$ and $X{(j)}$ are neighboring hospitals at positions i and j, distinguished by unique IDs.
Consensus processes are used to train the FLED-Block model using stored local models, enabling collaborative training. Proof of work allows data sharing among nodes. Consensus verifies local model quality by calculating Mean Prediction Accuracy Error (MPAE), reflecting predictive capability. All node data is encrypted using high random Chaotic public [58] and private keys [50, 52, 53] for confidentiality. MPAE is applied to all transactions, recorded in the blockchain’s distributed ledger.
Blockchain-Based Data Sharing Process
Security is crucial in data sharing between requester and source hospitals [59]. Instead of full data sharing, hospitals share only learned models with requesters. Hospitals communicate, and a consensus algorithm facilitates federated data learning. Provider and requester data are stored in blockchain nodes [60]. To maintain privacy, only learning models are shared, not original data [61]. In phase one, hospitals upload image datasets for collaborative learning. In phase two, hospitals share locally trained model weights with the blockchain and use federated learning to aggregate local models into global models.
Experimental Results
The proposed model was implemented using open-source TensorFlow Federated version 2.1.0, while classical models were developed using TensorFlow version 1.8 with Keras backend. Experiments were conducted on a PC workstation with an Intel Xeon CPU, NVIDIA Titan GPU, 16GB RAM, and 3.5 GHz operating frequency. Each dataset was split into 70% training, 20% testing, and 10% validation. 70% of each dataset (23,804, 31,501, and 2,443 images) were used for training the proposed model (TensorFlow Federated 2.1.0) and classical models (TensorFlow 1.8). Models were implemented on a federated blockchain using Python 3.9.1, with a CSS-based blockchain user interface. This setup validated and tested the algorithm. Model performance was evaluated using accuracy, precision, recall, specificity, and F1-score metrics, reflecting the model’s ability to differentiate between COVID-19 and non-COVID infections [62]. Table 2 lists mathematical expressions for performance metrics.
Table 2.
Performance Metrics Definitions for COVID-19 Detection Algorithms.
| Metric | Formula |
|---|---|
| Accuracy | (TP + TN) / (TP + TN + FP + FN) |
| Precision | TP / (TP + FP) |
| Recall | TP / (TP + FN) |
| Specificity | TN / (TN + FP) |
| F1-Score | 2 (Precision Recall) / (Precision + Recall) |
Note: TP = True Positive, TN = True Negative, FP = False Positive, FN = False Negative.
Medical diagnostic systems require high accuracy, precision, and recall. Early stopping [63] was used to mitigate overfitting and improve generalization, halting network training when validation performance showed no improvement for N consecutive epochs.
Results and Findings
The proposed architecture’s performance was validated in three stages. First, performance metrics were calculated for different CT datasets. Loss validation curves (LVCs) were generated to assess model performance. Finally, the algorithm’s superiority was demonstrated through comparisons with existing deep learning algorithms. Figures 6A–C show model performance across datasets.
Figure 6.
(A–C) Training and Validation Accuracy Curves for FLED-Block Model across Datasets 1, 2, and 3.
Alt Text (Figure 6A): Training and validation accuracy curves for the FLED-Block algorithm using Dataset 1, demonstrating model performance and convergence.
Alt Text (Figure 6B): Training and validation accuracy curves for the FLED-Block algorithm using Dataset 2, illustrating model accuracy with a larger dataset.
Alt Text (Figure 6C): Training and validation accuracy curves for the FLED-Block algorithm using Dataset 3, showing consistent model performance across different datasets.
Figures 6A–C display validation curves for training the proposed model with different datasets, while Figure 7 shows loss validation curves. Figure 6A shows a root mean square error (RMSE) of 0.001 between training and validation models. Similar characteristics are seen in Figure 6C. Dataset 2, being larger, shows slightly higher RMSE (0.0014 in Figure 6B). Average COVID-19 detection performance was 98.5% (Dataset 1), 98.3% (Dataset 2), and 98.5% (Dataset 3).
Figure 7.
Loss Validation Curves for FLED-Block Model across Datasets 1, 2, and 3.
Alt Text: Loss validation curves for the FLED-Block algorithm across Datasets 1, 2, and 3, demonstrating model loss reduction during training and validation.
Capsule feature extraction and ELM classification layers ensure consistent performance across multi-source heterogeneous datasets. Figure 8 presents performance metrics for different datasets, showing average accuracy of 98.4–98.5%, precision of 97–98%, and recall of 97.5–98%. The model exhibits high false alarm rates and low specificity across datasets.
Figure 8.
Performance Metrics for FLED-Block Model with Datasets 1, 2, and 3.
Alt Text: Performance metrics bar chart for the FLED-Block algorithm across Datasets 1, 2, and 3, comparing accuracy, precision, recall, specificity, and F1-score.
To demonstrate model excellence, comparative analyses were performed against deep learning algorithms like VGG-16/VGG-19 [64-66], AlexNETS [67], DenseNETS [68-70], ResNETS-50/100 [59, 71], and SegCAP [72]. Table 3 compares performance on Dataset 1. The proposed model outperformed SegCAP and Resnets-100.
Table 3.
Comparative Performance Analysis on Dataset 1.
| Algorithm | Accuracy | Precision | Recall | Specificity | F1-Score |
|---|---|---|---|---|---|
| VGG-16 | 0.8269 | 0.833 | 0.8234 | 0.170 | 0.832 |
| VGG-19 | 0.833 | 0.843 | 0.823 | 0.173 | 0.840 |
| Alexnets | 0.834 | 0.823 | 0.814 | 0.189 | 0.826 |
| Resnets-50 | 0.845 | 0.823 | 0.832 | 0.164 | 0.834 |
| Resnets-100 | 0.849 | 0.843 | 0.834 | 0.167 | 0.838 |
| Inception V3 | 0.80 | 0.82 | 0.821 | 0.190 | 0.801 |
| Densenets-121 | 0.82 | 0.83 | 0.834 | 0.167 | 0.812 |
| Desnsenet-119 | 0.78 | 0.793 | 0.80 | 0.200 | 0.80 |
| Densenets-150 | 0.81 | 0.802 | 0.794 | 0.80 | 0.73 |
| Mobilenets | 0.782 | 0.784 | 0.778 | 0.783 | 0.778 |
| SegCaps | 0.89 | 0.934 | 0.923 | 0.07 | 0.930 |
| Proposed model | 0.982 | 0.973 | 0.965 | 0.0335 | 0.970 |
Performance comparisons for Dataset 2 (Table 4) and Dataset 3 (Table 5) show similar trends. Increased dataset size slightly reduced performance for all models, but SegCAP, Resnets-100, and the proposed model maintained robust performance. The proposed model consistently outperformed others, demonstrating the effectiveness of ensembled capsule networks and ELMs for COVID-19 detection from diverse datasets.
Table 4.
Comparative Performance Analysis on Dataset 2.
| Algorithm | Accuracy | Precision | Recall | Specificity | F1-Score |
|---|---|---|---|---|---|
| VGG-16 | 0.797 | 0.783 | 0.7563 | 0.289 | 0.789 |
| VGG-19 | 0.732 | 0.743 | 0.723 | 0.273 | 0.7390 |
| Alexnets | 0.804 | 0.783 | 0.784 | 0.229 | 0.806 |
| Resnets-50 | 0.80 | 0.801 | 0.802 | 0.200 | 0.812 |
| Resnets-100 | 0.840 | 0.838 | 0.836 | 0.177 | 0.82 |
| Inception V3 | 0.678 | 0.677 | 0.675 | 0.675 | 0.681 |
| Densenets-121 | 0.790 | 0.784 | 0.779 | 0.221 | 0.79 |
| Desnsenet-119 | 0.777 | 0.781 | 0.78 | 0.229 | 0.774 |
| Densenets-150 | 0.80 | 0.792 | 0.789 | 0.728 | 0.73 |
| Mobilenets | 0.782 | 0.784 | 0.783 | 0.773 | 0.753 |
| SegCaps | 0.87 | 0.92 | 0.910 | 0.09 | 0.910 |
| ProposedModel | 0.982 | 0.973 | 0.965 | 0.335 | 0.970 |
Table 5.
Comparative Performance Analysis on Dataset 3.
| Algorithm | Accuracy | Precision | Recall | Specificity | F1-Score |
|---|---|---|---|---|---|
| VGG-16 | 0.8269 | 0.833 | 0.8234 | 0.170 | 0.832 |
| VGG-19 | 0.833 | 0.843 | 0.823 | 0.173 | 0.840 |
| Alexnets | 0.834 | 0.823 | 0.814 | 0.189 | 0.826 |
| Resnets-50 | 0.845 | 0.823 | 0.832 | 0.164 | 0.834 |
| Resnets-100 | 0.849 | 0.843 | 0.834 | 0.167 | 0.838 |
| Inception V3 | 0.80 | 0.82 | 0.821 | 0.190 | 0.801 |
| Densenets-121 | 0.82 | 0.83 | 0.834 | 0.167 | 0.812 |
| Desnsenet-119 | 0.78 | 0.793 | 0.80 | 0.200 | 0.80 |
| Densenets-150 | 0.81 | 0.802 | 0.794 | 0.80 | 0.73 |
| Mobilenets | 0.782 | 0.784 | 0.778 | 0.783 | 0.778 |
| SegCaps | 0.89 | 0.934 | 0.923 | 0.07 | 0.930 |
| ProposedModel | 0.982 | 0.973 | 0.965 | 0.335 | 0.970 |
Finally, comparisons with other blockchain-based learning models are presented in terms of detection accuracy, trust level, and dataset handling. Tables 6–8 compare the proposed model with blockchain-based learning models for data sharing and retrieval. The proposed model demonstrates superior suitability for blockchain integration, enabling efficient and secure data sharing and retrieval while maintaining data privacy and security.
Table 6.
Blockchain-Based Model Comparison on Dataset 1.
| References | Proposed model in blockchain | Number of cases | Average accuracy performance % | Trust level | Sharing and retrieval |
|---|---|---|---|---|---|
| Parnian et al. [40] | ResNETS | High | 89.5 | No | No |
| He et al. [41] | 2D-CNN | High | 85.4 | No | No |
| Rahimzadeh et al. [44] | Federated Capsule network learning | High | 91 | Medium | Yes |
| Ours | Federated Ensembled capsule networks | High | 98.5 | High | Yes |
Table 7.
Blockchain-Based Model Comparison on Dataset 2.
| References | Proposed model in blockchain | Number of cases | Average accuracy performance % | Trust level | Sharing and retrieval |
|---|---|---|---|---|---|
| Parnian et al. [40] | ResNETS | Very High | 88.4 | No | No |
| He et al. [41] | 2D-CNN | Very High | 83.3 | No | No |
| Rahimzadeh et al. [44] | Federated Capsule network learning | Very High | 89 | Medium | Yes |
| Ours | Federated Ensembled capsule networks | Very High | 98.5 | High | Yes |
Table 8.
Blockchain-Based Model Comparison on Dataset 3.
| References | Proposed model in blockchain | Number of cases | Average accuracy performance % | Trust level | Sharing and retrieval |
|---|---|---|---|---|---|
| Parnian et al. [40] | ResNETS | High | 89.5 | No | No |
| He et al. [41] | 2D-CNN | High | 85.4 | No | No |
| Rahimzadeh et al. [44] | Federated capsule network learning | High | 91 | Medium | Yes |
| Ours | Federated Ensembled capsule networks | High | 98.5 | High | Yes |
The proposed model and federated model by He et al. [41] show similar characteristics, but the proposed method offers a 7% trust increment due to chaotic encryption and improved detection accuracy from capsule and ELM integration.
Time and space complexity comparisons with state-of-the-art models are presented in Table 9. Time complexity is assessed using Big-O notation, O(n). Classical models on centralized systems exhibit O($n^{2n}$), while the proposed distributed model reduces complexity based on the number of nodes (5 in this experiment), decreasing N. Space complexity measures algorithm memory usage.
Table 9.
Time and Space Complexity Comparison with Other Learning Models.
| References | Proposed model in blockchain | Time complexity | Space complexity |
|---|---|---|---|
| Parnian et al. [40] | ResNETS | O($n^{2n}$) | 6.92 MB |
| He et al. [41] | 2D-CNN | O($n^{2n-1}$) | 5.54 MB |
| Rahimzadeh et al. [44] | Federated capsule network learning | O($n^{2n-5}$) | 3.25 MB |
| Ours | Federated ensembled capsule networks | O($n^{2n-5}$) | 2.85 MB |
The proposed federated learning model achieves lower time complexity due to its distributed nature and reduced space complexity due to the feed-forward nature of ELMs.
Conclusion
Traditional AI techniques for medical diagnosis often suffer from computational complexity and privacy vulnerabilities due to centralized data requirements. This paper introduces the FLED-Block framework, a blockchain-empowered federated approach, to enhance COVID-19 detection from heterogeneous CT images. FLED-Block facilitates secure data sharing among hospitals, maintaining privacy and security. The ensemble of capsule networks and extreme learning machines enables effective feature extraction and classification, improving COVID-19 detection accuracy across diverse datasets. Federated learning, coupled with blockchain technology and chaotic encryption keys, enhances data privacy and security. Comprehensive experimental results demonstrate the superior performance of the proposed model in terms of accuracy, precision, recall, and F1-score compared to other deep learning algorithms and blockchain-based federated models. While achieving high performance, future work will focus on improving real-time clinical database handling, reducing blockchain latency, and optimizing cost-effectiveness.
Data Availability Statement
The datasets analyzed during the current study are publicly available. References to the datasets are provided in the manuscript.
Ethics Statement
Ethical review and approval were not required for this study as it utilized publicly available de-identified datasets. Written informed consent was not applicable.
Author Contributions
RD conceived and designed the study. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare no conflict of interest.
Publisher’s Note
All claims in this article are solely those of the authors and do not represent affiliated organizations, publishers, editors, or reviewers. Product evaluations or claims are not guaranteed or endorsed by the publisher.
References
[List of references as in the original article]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
