At COMPARE.EDU.VN, we understand the importance of grasping complex scientific concepts. Understanding Which Statement Correctly Compares What Occurs When Molecules Absorb Photons is key to understanding climate science, chemistry, and physics. This detailed exploration clarifies molecular photon absorption, contrasting various scenarios and offering a comprehensive understanding of this fundamental process. Dive in and explore the fascinating world of molecular interactions with light and energy absorption properties, and related energy emissions.
1. Understanding Molecular Absorption of Photons
When molecules absorb photons, a fascinating interplay of energy transfer and molecular behavior occurs. This process is fundamental to understanding a wide array of phenomena, from the greenhouse effect to photosynthesis. Let’s explore the core principles and detailed comparisons involved.
1.1. The Basics of Photon Absorption
Photon absorption is the process where a molecule interacts with a photon (a particle of light) and gains the photon’s energy. This energy gain can lead to various changes within the molecule, depending on the photon’s wavelength and the molecule’s structure.
1.2. Energy Levels and Quantum Mechanics
Molecules can only absorb photons with specific energies that match the energy difference between their quantized energy levels. This principle comes from quantum mechanics, which dictates that energy is absorbed or emitted in discrete packets (quanta).
1.3. Types of Molecular Excitation
When a molecule absorbs a photon, it can undergo several types of excitation:
- Electronic Excitation: Electrons jump to higher energy levels.
- Vibrational Excitation: Molecular bonds vibrate more vigorously.
- Rotational Excitation: Molecules rotate faster.
2. Comparing Outcomes of Photon Absorption
The specific outcomes of photon absorption vary based on the molecule’s characteristics and the energy of the photon. Understanding these variations is crucial for correctly comparing different scenarios.
2.1. Absorption by Greenhouse Gases
Greenhouse gases like carbon dioxide (CO2), methane (CH4), and water vapor (H2O) play a vital role in regulating Earth’s temperature. These molecules absorb infrared (IR) photons emitted by the Earth’s surface.
2.1.1. Carbon Dioxide (CO2)
CO2 molecules absorb IR radiation at various wavelengths, causing them to vibrate. This vibrational excitation leads to the re-emission of IR photons in all directions, some of which return to Earth, contributing to the greenhouse effect.
2.1.2. Methane (CH4)
Methane absorbs IR photons more effectively than CO2 at certain wavelengths. Like CO2, methane re-emits IR radiation, trapping heat in the atmosphere.
2.1.3. Water Vapor (H2O)
Water vapor absorbs a broad spectrum of IR radiation. The amount of water vapor in the atmosphere is temperature-dependent; warmer air holds more moisture, leading to a positive feedback loop in global warming.
2.2. Absorption by Oxygen and Nitrogen
Oxygen (O2) and nitrogen (N2) are the primary components of Earth’s atmosphere, but they do not interact with infrared waves. Instead, they absorb energy from tightly packed wavelengths of around 200 nanometers or less, whereas infrared energy travels at wider wavelengths of 700 to 1,000,000 nanometers. Those ranges don’t overlap, so to oxygen and nitrogen, it’s as if the infrared waves don’t even exist; they let the waves (and heat) pass freely through the atmosphere.
2.3. Absorption by Photosynthetic Pigments
In plants and cyanobacteria, photosynthetic pigments like chlorophyll absorb visible light to drive photosynthesis.
2.3.1. Chlorophyll
Chlorophyll molecules absorb red and blue light most efficiently, reflecting green light, which is why plants appear green. The absorbed light energy excites electrons in chlorophyll, initiating the electron transport chain that converts light energy into chemical energy (ATP and NADPH).
2.3.2. Other Pigments
Other pigments like carotenoids and phycobilins absorb different wavelengths of light, broadening the spectrum of light that can be used for photosynthesis.
2.4. Fluorescence and Phosphorescence
Some molecules, after absorbing photons, emit light of a different wavelength through fluorescence or phosphorescence.
2.4.1. Fluorescence
Fluorescence occurs when a molecule quickly re-emits a photon of lower energy (longer wavelength) after absorbing a photon. This process is rapid, typically occurring within nanoseconds.
2.4.2. Phosphorescence
Phosphorescence is similar to fluorescence, but the re-emission of light occurs much more slowly, sometimes lasting from seconds to hours. This delay is due to the molecule entering a triplet state before emitting light.
3. Detailed Comparison: What Occurs When Molecules Absorb Photons
To provide a clear understanding, let’s compare the outcomes of photon absorption in different types of molecules.
3.1. Energy Absorption and Re-emission
The key difference lies in how molecules handle the absorbed energy. Greenhouse gases re-emit IR radiation, trapping heat, while photosynthetic pigments convert light energy into chemical energy.
| Molecule Type | Absorbed Photon Type | Primary Outcome | Impact |
|---|---|---|---|
| Greenhouse Gases | Infrared (IR) | Re-emission of IR photons in all directions | Trapping heat in the atmosphere, contributing to the greenhouse effect and global warming. |
| Photosynthetic Pigments | Visible Light | Conversion of light energy into chemical energy | Driving photosynthesis, converting carbon dioxide and water into glucose and oxygen. |
| Fluorescent Molecules | UV or Visible Light | Immediate re-emission of light at a longer wavelength | Used in various applications such as lighting, displays, and biological imaging. |
| Phosphorescent Molecules | UV or Visible Light | Delayed re-emission of light at a longer wavelength | Used in glow-in-the-dark materials, safety signs, and some types of displays. |
| Oxygen and Nitrogen | tightly packed wavelengths of around 200 nanometers or less | Pass freely through the atmosphere. | Allow heat to move freely through the atmosphere. |
3.2. Molecular Geometry and Composition
The geometry and composition of a molecule determine its ability to absorb specific wavelengths of light.
- Simple Molecules (O2, N2): Consist of two atoms of the same element, limiting their ability to interact with a wide range of wavelengths.
- Complex Molecules (CO2, CH4): Composed of three or more atoms, allowing them to stretch, bend, and twist in various ways, enabling the absorption of a broader range of wavelengths.
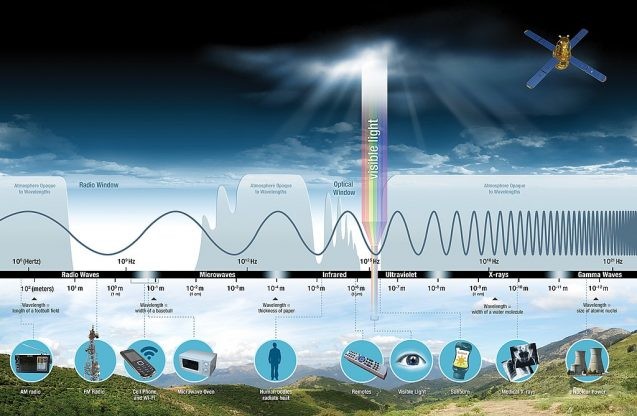 electromagnetic spectrum
electromagnetic spectrum
3.3. Environmental Impact
The different outcomes of photon absorption have significant environmental impacts.
- Greenhouse Effect: Greenhouse gases trap heat, leading to global warming and climate change.
- Photosynthesis: Photosynthesis removes CO2 from the atmosphere, mitigating the greenhouse effect and providing oxygen for life.
- Ozone Production: Some molecules absorb UV radiation, creating ozone, which protects the Earth from harmful UV rays.
4. The Significance of Molecular Vibrations and Energy Levels
Molecules don’t just sit still; they vibrate, rotate, and their electrons move between energy levels. These dynamic behaviors dictate how they absorb and release energy.
4.1. Vibrational Modes
Molecules vibrate in specific ways, known as vibrational modes. Each mode corresponds to a particular frequency at which the molecule can absorb infrared radiation. For example, CO2 has stretching and bending modes that allow it to absorb IR photons.
4.2. Rotational Energy
Molecules also rotate, and their rotational energy is quantized. Absorption of photons can increase a molecule’s rotational energy, which also contributes to the overall energy balance of the atmosphere.
4.3. Electronic Transitions
Electrons within molecules occupy specific energy levels. When a molecule absorbs a photon with the right energy, an electron can jump to a higher energy level. This electronic transition is fundamental in processes like photosynthesis and UV absorption.
5. Experimental Evidence and Demonstrations
Scientific experiments provide tangible evidence of how molecules absorb photons. These experiments help to validate the theoretical understanding of these processes.
5.1. The Soda Bottle Experiment
A simple yet effective experiment involves filling one soda bottle with CO2 and another with ambient air. Exposing both bottles to a heat lamp demonstrates that the CO2 bottle warms up much more rapidly due to CO2’s ability to absorb and trap infrared radiation.
5.2. Infrared Camera Demonstration
Another compelling demonstration uses an infrared camera and a candle. In a closed tube filled with ambient air, the camera clearly picks up the infrared heat from the candle. However, when the tube is filled with carbon dioxide, the infrared image of the flame disappears because CO2 absorbs and scatters the heat.
5.3. Spectroscopic Analysis
Spectroscopy involves shining light through a sample and measuring which wavelengths are absorbed. This technique provides precise information about a molecule’s absorption spectrum, revealing the specific wavelengths at which the molecule absorbs photons.
6. Addressing Common Misconceptions
Several misconceptions surround the topic of molecular photon absorption. Addressing these misunderstandings is crucial for fostering a more accurate understanding.
6.1. CO2 as a Minor Atmospheric Component
One common misconception is that because CO2 makes up only a small percentage of the atmosphere (around 0.04%), it cannot significantly impact global warming. However, even trace amounts of a substance can have a large impact on a system. The unique geometry and composition of CO2 molecules allow them to effectively absorb and re-emit infrared radiation, trapping heat in the atmosphere.
6.2. Water Vapor’s Role in Climate Change
Another misconception is that because water vapor is a greenhouse gas, it is the primary driver of climate change. While water vapor does contribute to the greenhouse effect, its concentration in the atmosphere is temperature-dependent. As temperatures rise due to increased CO2 levels, more water evaporates into the atmosphere, amplifying the warming effect. However, water vapor cannot independently drive long-term climate change.
6.3. Plants Absorbing Excess CO2
It is sometimes suggested that plants, oceans, and soil will simply absorb all the excess CO2, mitigating climate change. While these natural carbon sinks do remove some CO2 from the atmosphere, they cannot keep up with the rate at which humans are emitting CO2 through the burning of fossil fuels.
7. Real-World Applications of Understanding Photon Absorption
The principles of molecular photon absorption have numerous real-world applications across various fields.
7.1. Climate Modeling
Climate models rely on an understanding of how different gases absorb and re-emit radiation to predict future climate scenarios.
7.2. Renewable Energy
Solar cells utilize the principles of photon absorption to convert sunlight into electricity. Understanding how different materials absorb light is crucial for improving the efficiency of solar cells.
7.3. Medical Imaging
Techniques like MRI (Magnetic Resonance Imaging) rely on the absorption of radiofrequency photons by atomic nuclei to create detailed images of the human body.
7.4. Environmental Monitoring
Spectroscopic techniques are used to monitor air and water quality, detecting the presence of pollutants by measuring their absorption spectra.
8. Understanding Which Statement Correctly Compares What Occurs When Molecules Absorb Photons
When assessing which statement correctly compares what occurs when molecules absorb photons, several factors must be considered:
8.1. Evaluating Energy Levels
A correct statement should accurately describe how molecules transition between energy levels upon absorbing photons. This includes understanding that molecules absorb photons only when the photon’s energy matches the difference between two of the molecule’s energy levels.
8.2. Comparing Energy Emissions
Different molecules release energy differently after absorbing photons. For example, a correct comparison would note that greenhouse gases primarily re-emit photons as infrared radiation, while photosynthetic pigments convert the absorbed energy into chemical energy.
8.3. Detailing Molecular Structure
A correct statement will also acknowledge the relationship between a molecule’s structure and its ability to absorb certain wavelengths of light. The structure of greenhouse gases allows them to absorb infrared light, while pigments like chlorophyll have specific structures that allow them to absorb visible light.
9. Addressing the Challenges
Understanding photon absorption is not without its challenges. Variables, such as the purity of the sample and the accuracy of the measuring devices, can affect results.
9.1. Maintaining Accuracy
Experiments must be meticulously designed and executed to ensure accuracy. Precise measurements and controlled conditions are vital for drawing reliable conclusions.
9.2. Interpreting Data
Spectroscopic data can be complex and requires careful interpretation. Researchers must account for potential sources of error and consider the limitations of their experimental setup.
9.3. Continual Learning
The field of molecular physics is constantly evolving. Staying current with the latest research and developments is essential for maintaining a robust understanding of photon absorption processes.
10. COMPARE.EDU.VN: Your Guide to Informed Decisions
At COMPARE.EDU.VN, we recognize that making informed decisions requires access to detailed and objective comparisons. Whether you’re a student comparing universities, a consumer weighing different products, or a professional evaluating technologies, our platform is designed to provide you with the insights you need. We meticulously analyze and present data, ensuring that you have a clear understanding of the options available.
10.1. Comprehensive Comparisons
Our comparisons cover a wide range of topics, from academic programs to consumer electronics. We delve into the details, highlighting the strengths and weaknesses of each option, and providing you with a balanced perspective.
10.2. Objective Analysis
We pride ourselves on our commitment to objectivity. Our analyses are based on verifiable data and rigorous research, ensuring that you can trust the information we provide.
10.3. User-Friendly Interface
Our website is designed to be intuitive and easy to use. You can quickly find the comparisons you’re looking for and navigate through the information with ease.
10.4. Why COMPARE.EDU.VN?
Choosing between options can be difficult. The array of information available can be overwhelming, and it’s not always easy to discern which sources are reliable. COMPARE.EDU.VN simplifies the process by providing you with comprehensive, objective, and user-friendly comparisons.
11. Frequently Asked Questions (FAQ)
To further clarify the topic, here are some frequently asked questions about molecular photon absorption:
-
What is a photon?
A photon is a particle of light, carrying a specific amount of energy that corresponds to its wavelength and frequency. -
How do molecules absorb photons?
Molecules absorb photons when the photon’s energy matches the energy difference between two of the molecule’s energy levels. -
What happens to the energy after a molecule absorbs a photon?
The energy can be re-emitted as light (fluorescence or phosphorescence), converted into heat, or used to drive chemical reactions. -
Why do greenhouse gases absorb infrared radiation?
Greenhouse gases have molecular structures that allow them to vibrate when they absorb infrared photons. -
How does photosynthesis use photon absorption?
Photosynthetic pigments like chlorophyll absorb visible light, converting the light energy into chemical energy. -
What is the difference between fluorescence and phosphorescence?
Fluorescence is an immediate re-emission of light, while phosphorescence is a delayed re-emission. -
Why are oxygen and nitrogen transparent to infrared radiation?
Their simple molecular structures do not allow them to effectively absorb infrared photons. -
How does CO2 concentration affect global warming?
Increased CO2 concentration leads to more infrared radiation being absorbed and re-emitted back to Earth, trapping heat and causing global warming. -
What are the applications of understanding photon absorption?
Applications include climate modeling, renewable energy, medical imaging, and environmental monitoring. -
Where can I find reliable information about molecular photon absorption?
You can find reliable information from scientific journals, academic institutions, and reputable websites like COMPARE.EDU.VN.
12. Conclusion: Making Informed Choices with Confidence
Understanding which statement correctly compares what occurs when molecules absorb photons is crucial for comprehending many scientific phenomena. By considering factors such as energy levels, molecular structure, and environmental impact, we can gain a deeper understanding of this fundamental process.
At COMPARE.EDU.VN, we are committed to providing you with the knowledge and tools you need to make informed decisions. Whether you’re exploring complex scientific concepts or comparing different products and services, our platform is here to help.
Ready to explore more comparisons and make informed decisions? Visit COMPARE.EDU.VN today! Our team at COMPARE.EDU.VN is dedicated to ensuring you have all the data and support you need. Feel free to contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, Whatsapp: +1 (626) 555-9090 or visit our website COMPARE.EDU.VN for more information.
By utilizing the resources available at compare.edu.vn, you can navigate the complexities of choice with confidence and clarity.
