Which Of These Membranes Is Dry Compared To The Others? COMPARE.EDU.VN offers an in-depth analysis of membrane dryness and related features, providing a comprehensive overview for informed decisions, with comparisons, dryness evaluation, and material science insights. Navigate the complexities of membrane properties with ease, enhancing your understanding of material characteristics, dryness levels, and surface analysis on COMPARE.EDU.VN.
1. Introduction: Understanding Membrane Dryness
Ion-exchange membranes (IEMs) are increasingly crucial in diverse applications, from water treatment to energy storage. The efficiency and success of these applications significantly depend on the specific properties of the membranes used. Among these properties, dryness—or rather, the water content and behavior—plays a vital role in determining the membrane’s performance and longevity. This article delves into a comprehensive comparison of various IEMs, focusing on their water content and how it affects their overall functionality. Specifically, we will explore the dryness characteristics of several commercially available and novel membranes, providing insights into which ones tend to be drier compared to others under similar conditions.
1.1 The Importance of Membrane Properties
Membrane technologies are revolutionizing traditional methods of purification, concentration, conditioning, and separation of substances. These environmentally friendly technologies are finding applications in wastewater treatment, extraction of antioxidants, and recovery of valuable medicinal products. IEMs, however, constitute a significant portion of the cost in electrodialysis (ED) devices, sometimes reaching up to 50% of the total expense. Therefore, the transport properties and cost of IEMs are key determinants in the implementation of these technologies.
1.2 Types of Ion-Exchange Membranes
Traditionally, IEMs are classified into two main types:
- Homogeneous Membranes: These have a uniform structure at the nanoscale level (up to 100 nm) and can be single-phase or two-phase. Examples include Nafion and Neosepta AMX and CMX.
- Heterogeneous Membranes: These consist of micrometer-sized ion-exchange polymer granules incorporated into an inert binder. Examples include MK-40 and MA-40.
In recent years, a new type of IEM has emerged, based on a three-dimensional structure of nanofibers made using the electrospinning method. These electro-spun nanofiber ion exchange membranes (EN-IEMs) offer a novel approach to membrane design, combining the roles of an inert filler and a reinforcing material.
1.3 Challenges and Opportunities
Despite the potential of EN-IEMs, their widespread adoption is hindered by a lack of comprehensive data on their transport characteristics. Existing research has primarily focused on electrical conductivity and surface modification. Therefore, a thorough understanding of the structural and transport properties of these membranes is essential for their effective application in dialysis, electrodialysis, and other membrane-based processes. This is where COMPARE.EDU.VN steps in, offering detailed comparisons and analyses to guide informed decisions.
2. Understanding Membrane Structure and Materials
To effectively compare the dryness of different IEMs, it is essential to understand their structural characteristics and the materials used in their construction.
2.1 Composition of Fujifilm Membranes
Fujifilm membranes, produced using the electrospinning method, consist of a three-dimensional structure of inert polyolefin fibers. These fibers are formed from a polymer solution in an organic solvent under the action of a strong electric field. The aerogel formed by these fibers is then pressed to a specific thickness, and the space between the fibers is filled with aliphatic polyamide (PA) functionalized with either quaternary ammonium bases (for anion-exchange membranes) or sulfo groups (for cation-exchange membranes).
2.2 Homogeneous Membranes (Astom)
Homogeneous membranes such as AMX and CMX are made using the “paste method.” This involves a mixture of styrene monomer with functional groups, divinylbenzene as a crosslinking agent, a radical polymerization initiator, and powdered polyvinyl chloride (PVC). The paste is deposited on a reinforcing PVC fabric, and copolymerization is carried out before sulfonation (for CEMs) or amination (for AEMs).
2.3 Heterogeneous Membranes (Shchekinoazot)
Heterogeneous membranes like MA-41 and MK-40 are made by hot rolling low-pressure polyethylene (PE) with AV-17 and KU-2 ion-exchange resin powders, respectively. A reinforcing nylon mesh is introduced by hot pressing to enhance mechanical strength.
2.4 Summary of Materials and Manufacturing Methods
The following table summarizes the basic materials and manufacturing methods for the different types of membranes:
| Membranes | Type | Ion Exchange Matrix | Fixed Groups | Inert Binder | Reinforcing Material | Manufacturing Method |
|---|---|---|---|---|---|---|
| MA-41 | Heterogeneous | DVB + PS | * | PE | Nylon Mesh | Hot Rolling |
| MK-40 | Heterogeneous | DVB + PS | ** | PE | Nylon Mesh | Hot Rolling |
| AMX | Homogeneous | DVB + PS | * | PVC | PVC Fabric | “Paste” Method |
| CMX | Homogeneous | DVB + PS | ** | PVC | PVC Fabric | “Paste” Method |
| AEM Type-I, AEM Type-II, AEM Type-Х | EN-IEM | PA | * | – | 3D Polyolefin Fibers Structure | Filling the voids of the 3D fiber structure with ion-exchange material |
| CEM Type-I, CEM Type-II, CEM Type-Х | EN-IEM | PA | ** | – | 3D Polyolefin Fibers Structure | Filling the voids of the 3D fiber structure with ion-exchange material |
*Mainly quaternary ammonium bases, –(CH3)3N+, and a small amount of secondary and tertiary amines.
**Sulfo group –SO3−.
DVB + PS is a copolymer of polystyrene and divinylbenzene; PE is low-pressure polyethylene; PA is polyamide; PVC is polyvinyl chloride.
2.5 Key Characteristics of Swollen Membranes
The following table presents some key characteristics of the swollen membranes:
| Membranes | Thickness of Air-Dried Membrane, Microns | *Thickness of Wet Membrane, Microns | Exchange Capacity, mmol g−1 wet | Density, g cm−3 wet | Water Content, g Н2О/g wet, % | Water Content, mol H2O/mol Functional Groups |
|---|---|---|---|---|---|---|
| Cation-Exchange | ||||||
| CEM Type-I | 120 ± 5 | 140 ± 10 | 1.43 ± 0.05 | 1.15 | 29 ± 5 | 11.3 ± 1 |
| CEM Type-II | 165 ± 5 | 180 ± 10 | 1.35 ± 0.05 | 1.13 | 25 ± 2 | 10.3 ± 1 |
| CEM Type-X | 125 ± 5 | 130 ± 5 | 1.67 ± 0.05 | 1.20 | 21 ± 5 | 7 ± 1 |
| CMX | 155 ± 5 | 170 ± 5 | 1.61 ± 0.05 | 1.32 | 22 ± 3 | 8 ± 1 |
| MK-40 | 440 ± 10 | 520 ± 20 | 1.43 ± 0.08 | 1.18 | 26 ± 5 | 10 ± 1 |
| Anion-Exchange | ||||||
| AEM Type-I | 120 ± 5 | 125 ± 5 | 1.50 ± 0.05 | 1.06 | 8 ± 2 | 3.3 ± 1 |
| AEM Type-II | 165 ± 5 | 175 ± 5 | 1.08 ± 0.05 | 1.06 | 10 ± 5 | 5.5 ± 1 |
| AEM Type-X | 115 ± 5 | 120 ± 5 | 1.50 ± 0.05 | 1.08 | 23 ± 2 | 8.7 ± 1 |
| AMX | 125 ± 5 | 135 ± 5 | 1.23 ± 0.05 | 1.22 | 14 ± 2 | 6.5 ± 1 |
| MA-41 | 430 ± 10 | 450 ± 50 | 1.22 ± 0.06 | 1.06 | 30 ± 2 | 8.7 ± 1 |
*Membrane equilibrated with 0.02 M NaCl solution.
3. Methods of Membrane Characterization
Before conducting measurements, all membrane samples undergo standard salt preparation in NaCl solutions. Several methods are used to characterize the membranes:
3.1 Total Exchange Capacity
The total exchange capacity (Qsw) is determined by the static acid-base method. This involves equilibrating the membrane with a known concentration of acid or base and then titrating to determine the amount of acid or base absorbed.
3.2 Water Content
Water content (W, %) of the membranes is determined as the mass ratio of water to swollen membrane by the gravimetric method. This involves weighing the membrane in its swollen state and then drying it completely to determine the mass of water lost.
3.3 Specific Water-Absorbing Capacity
The specific water-absorbing capacity (the number of moles of water per one mole of the fixed groups of the swollen membrane, nw) is determined by the formula:
| nw= WPH2O·Q |
where PH2O is the molar mass of water, equal to 18 g/mol; Q is the membrane exchange capacity, mol/gw.
3.4 Membrane Thickness
The thickness of the swollen membrane (d, μm) is controlled by a high-precision digital micrometer with an accuracy of 1 μm. The membrane thickness is obtained by averaging the results of 10 measurements made at various points of the sample under study.
3.5 Water Contact Angles
The water contact angles of the swollen membrane surface are determined by the method of a resting drop. The distilled water serves as a test liquid, and the contact angles are recorded 20 seconds after the test drop touches the surface.
3.6 Visualization Techniques
Visualization of the surface and volume of swollen IEM is carried out using an optical microscope equipped with a digital eyepiece USB camera. To increase the image contrast, Fujifilm membranes are placed in a solution containing a high molecular weight dye (anthocyanin) for 24 hours before the measurements.
3.7 Morphology and Chemical Composition
The morphology and chemical composition of the air-dry IEM surface are studied using AFM and SEM, equipped with an energy dispersive spectrometer (EDS) and the device for X-ray fluorescence elemental spectroscopy (XRF).
3.8 Specific Electric Conductivity
The specific electric conductivity of IEM (κ*) is determined by a differential method using a clip cell and an immittance meter at an alternating current frequency of 1 kHz.
The electric conductivity of membranes (κ*) is determined by the equation:
| κ*=dmRm+s−Rs |
where Rm+s is the resistance of membrane and solution; Rs is the resistance of solution; and dm is the membrane thickness in the solution of given concentration.
3.9 Diffusion Characteristics
The diffusion characteristics of the IEM are investigated using a two-chamber flow cell. The membrane separates two tracts: distilled water is pumped through one of them, and a NaCl solution of a given concentration is pumped through the other tract.
3.10 Transport Numbers
The transport numbers of counterions (t1*) and co-ions (tA*) in the membranes under study are obtained using the concentration dependences of the specific conductivity (κ*) and the integral diffusion permeability coefficient of the membranes (P).
| t1*=12+14−P*F2c2RTκ* |
| tA*=1−t1* |
where F is the Faraday constant; R is the gas constant, T is the temperature; C is the electrolyte concentration (NaCl), P* is the local diffusion permeability coefficient of the membrane.
3.11 Standard Contact Porosimetry
The standard contact porosimetry is used to estimate the integral volume of sorbed water (V) and the number of water molecules per AEM fixed group as functions of the effective pore radius (r).
4. Results and Discussions
4.1 Structural Characteristics
SEM and AFM images of EN-IEM air-dry samples show randomly arranged reinforcing fibers of thickness from 10 to 25 microns, occupying a significant part of the EN-IEM volume. Some of them protrude slightly above the surface. Round holes, with diameters varying from 3 to 25 microns, are observed on the surfaces of all investigated membranes.
Optical microscopy data indicate that extended macropores exist in some samples of EN-IEM (Type-I, Type-II) at the interface of the polymer fiber with the ion-exchange material. These pores are filled with an optically transparent equilibrium solution in the case of swollen samples in equilibrium with sodium chloride solution.
The elemental composition of the investigated EN-IEMs, obtained using XRF, indicates that the reinforcing fibers correspond to polyolefin, while the ion-exchange material corresponds to polyamide. The percentage of nitrogen and chlorine (in AEMs), as well as sulfur (in CEMs), increases in proportion to the growth in the exchange capacity of dry membranes.
Both the studied heterogeneous membranes and EN-IEMs contain extended macropores, which are formed at the points of contact of the fibers (Type-I and Type-II) and the reinforcing net (MA-41, MK-40) with the ion-exchange material. No such pores are observed in the AMX and CMX membranes.
4.2 Specific Conductivity
The concentration dependences of the specific conductivity of the investigated membranes in sodium chloride solutions show that the values of κ¯ increase in the series CEM Type-II < MK-40 < CMX ≈ CEM Type-I < CEM Type-X (for CEMs) and AMX ≈ AEM Type-II < MA-41 < AEM Type-X < AEM Type-I (for AEMs) in the same order as the exchange capacity of the gel phase.
The AEM Type I membrane exhibits greater κ¯ and D1¯ values compared to AEM Type-X, which may be attributed to a higher content of secondary and tertiary amino groups in the AEM Type-I membrane.
4.3 Diffusion Permeability
The integral diffusion permeability coefficient, P, is mainly determined by the rate of co-ion diffusion, which depends on the gel phase exchange capacity (Q¯), the co-ion concentration (C¯A) and diffusion coefficient (D¯A) in this phase, the volume fraction of electroneutral solution (f2), and the relative disposition of the gel and intergel solution phases, parameter α. The value of P is the lowest for the membranes with small f2 and is essentially higher for the MK-40, MA-41, and Type I cation- and anion-exchange Fujifilm membranes.
4.4 Counterion and Co-ion Transport Numbers
The concentration dependences of the real transport numbers (t1*) of counterions in the investigated IEMs indicate that the permselectivity of the CEM Type-II and CEM Type-X cation-exchange membranes and the AEM Type-II and AEM Type-X anion-exchange membranes is close to that determined for the homogeneous CMX and AMX membranes. The permselectivity of the Type-I cation- and anion-exchange membranes is comparable to that of heterogeneous membranes MK-40, MA-41.
5. Comparing Membrane Dryness
Based on the data presented, we can now compare the relative dryness of the different IEMs. The key factor influencing dryness is the water content and the specific water-absorbing capacity.
5.1 Water Content Comparison
Looking at the water content data in Table 2, we can make the following observations:
- AEM Type-I exhibits the lowest water content at 8 ± 2%, indicating it is the driest among the anion-exchange membranes.
- AEM Type-II has a slightly higher water content at 10 ± 5%.
- AMX shows a water content of 14 ± 2%, higher than both AEM Type-I and AEM Type-II.
- AEM Type-X has the highest water content among the Fujifilm AEMs, at 23 ± 2%.
- MA-41 has the highest water content among all membranes at 30 ± 2%.
- CEM Type-X has the lowest water content at 21 ± 5% among the CEM membranes, while CEM Type-II has slightly more water content at 25 ± 2%.
- CEM Type-I has the most water content at 29 ± 5% among all the CEM membranes.
- CMX has a water content of 22 ± 3%.
- MK-40 has a water content of 26 ± 5%.
5.2 Specific Water-Absorbing Capacity
The specific water-absorbing capacity (nw) provides insights into how many water molecules are associated with each functional group in the membrane. Membranes with lower nw values are generally considered drier.
- AEM Type-I has the lowest nw value at 3.3 ± 1, confirming its drier nature.
- AEM Type-II has an nw value of 5.5 ± 1.
- AMX has an nw value of 6.5 ± 1.
- AEM Type-X has an nw value of 8.7 ± 1.
- MA-41 has an nw value of 8.7 ± 1.
- CEM Type-X has an nw value of 7 ± 1.
- CEM Type-II has an nw value of 10.3 ± 1.
- CEM Type-I has an nw value of 11.3 ± 1.
- CMX has an nw value of 8 ± 1.
- MK-40 has an nw value of 10 ± 1.
5.3 Impact of Macropores
The presence of macropores in heterogeneous membranes and EN-IEMs also affects their water retention and overall dryness. Membranes with a higher volume fraction of macropores (higher f2) tend to have lower water content in the gel phase but may exhibit higher overall water uptake due to the presence of free water in the pores.
5.4 The Role of Hydrophilic Aliphatic Polyamide
The Fujifilm membranes’ unique properties are based on hydrophilic aliphatic polyamide (PA), from which the three-dimensional structure of interwoven fibers is created. It is noteworthy that both the AEM Type-I and the CEM Type-X show the highest and the lowest permeabilities.
5.5 Key Factors Affecting Water Content
- Functional Group Type and Density: Sulfonic acid groups in CEMs and quaternary ammonium groups in AEMs have different affinities for water. Higher density of these groups generally leads to higher water content.
- Polymer Matrix: The chemical nature of the polymer matrix (aliphatic polyamide vs. styrene-divinylbenzene copolymer) influences water uptake.
- Macropores: The presence and volume fraction of macropores affect water retention.
- Manufacturing Process: The electrospinning method used to create Fujifilm membranes results in a unique structure that influences water content and transport properties.
5.6 Summary
The AEM Type-I membrane stands out as being relatively dry compared to the other membranes studied. It has a considerably lower water content and specific water-absorbing capacity than other membranes, including the AEM Type-II, AMX, and MA-41. This dryness is likely due to the membrane’s unique structure, lower density of hydrophilic functional groups, and the properties of the polymer matrix.
6. Practical Implications
Understanding the relative dryness of IEMs has significant practical implications for various applications:
6.1 Electrodialysis
In electrodialysis, membrane dryness can affect ion transport efficiency and energy consumption. Drier membranes may exhibit lower ionic conductivity but higher selectivity, while wetter membranes may have higher conductivity but lower selectivity. The optimal dryness level depends on the specific application and operating conditions.
6.2 Fuel Cells
In fuel cells, membrane dryness is critical for maintaining proton conductivity and preventing fuel crossover. Membranes that are too dry may exhibit reduced proton conductivity, while those that are too wet may suffer from fuel crossover, reducing fuel cell efficiency.
6.3 Water Treatment
In water treatment applications, membrane dryness can affect fouling resistance and long-term performance. Drier membranes may be less susceptible to fouling by organic compounds, while wetter membranes may exhibit better water permeability.
6.4 Dialysis
For dialysis applications, the membrane’s water content can influence the transport of small mineral ions (Na+, Cl–), as well as high-molecular compounds (polyphenols, amino acids, peptides, polysaccharides, and others).
6.5 Further Insights with COMPARE.EDU.VN
For those looking to make informed decisions about IEM selection, COMPARE.EDU.VN offers detailed comparisons and analyses. By providing a platform for evaluating membrane properties, we aim to assist researchers and engineers in choosing the right membranes for their specific needs. This is where COMPARE.EDU.VN bridges the gap, offering structured comparisons and detailed analytics for informed choices.
7. Conclusions
The comparative analysis of cation- and anion-exchange membranes from various manufacturers reveals significant differences in their water content, specific water-absorbing capacity, and overall dryness. The microheterogeneous model effectively describes the structure-property relationships of these membranes.
Among the membranes studied, the AEM Type-I membrane stands out as being relatively dry due to its lower water content and specific water-absorbing capacity. The dryness of IEMs is influenced by several factors, including the type and density of functional groups, the polymer matrix, the presence of macropores, and the manufacturing process.
Understanding the relative dryness of IEMs is crucial for optimizing their performance in various applications, including electrodialysis, fuel cells, and water treatment. As membrane technologies continue to evolve, further research is needed to develop IEMs with tailored dryness levels for specific applications.
8. FAQs
Q1: What is an ion-exchange membrane (IEM)?
A: An ion-exchange membrane is a semi-permeable membrane that allows the selective transport of ions, either cations (positive ions) or anions (negative ions), while blocking the passage of other ions or molecules.
Q2: Why is membrane dryness important?
A: Membrane dryness (or water content) affects various properties, including ionic conductivity, selectivity, mechanical strength, and resistance to fouling. Optimizing dryness is crucial for achieving high performance in specific applications.
Q3: What are the main types of ion-exchange membranes?
A: The main types include homogeneous membranes (uniform structure), heterogeneous membranes (ion-exchange particles in a binder), and electro-spun nanofiber ion exchange membranes (EN-IEMs) made using the electrospinning method.
Q4: What factors affect the dryness of a membrane?
A: Factors include the type and density of functional groups (e.g., sulfonic acid, quaternary ammonium), the polymer matrix, the presence of macropores, and the manufacturing process.
Q5: Which of the membranes studied is relatively dry compared to the others?
A: The AEM Type-I membrane exhibits lower water content and specific water-absorbing capacity, indicating it is relatively dry compared to the other membranes studied.
Q6: How is membrane dryness measured?
A: Membrane dryness is typically measured by determining the water content using gravimetric methods and specific water-absorbing capacity. Other techniques include contact angle measurements and porosimetry.
Q7: What is the microheterogeneous model?
A: The microheterogeneous model is a theoretical framework used to describe the structure and properties of IEMs. It considers the membrane as a two-phase system consisting of a gel phase (polymer matrix and ions) and an intergel solution phase (electroneutral solution).
Q8: How do macropores affect membrane dryness?
A: Macropores can affect water retention and transport properties. Membranes with a higher volume fraction of macropores may have lower water content in the gel phase but higher overall water uptake due to free water in the pores.
Q9: What are the practical implications of membrane dryness in electrodialysis?
A: In electrodialysis, membrane dryness affects ion transport efficiency and energy consumption. Drier membranes may exhibit lower ionic conductivity but higher selectivity, while wetter membranes may have higher conductivity but lower selectivity.
Q10: Where can I find more detailed comparisons of ion-exchange membranes?
A: You can find detailed comparisons and analyses of ion-exchange membranes at COMPARE.EDU.VN. This website provides comprehensive data and insights to help you make informed decisions.
9. Call to Action
Are you struggling to compare different ion-exchange membranes for your specific application? Do you need detailed, objective information to make an informed decision? Visit COMPARE.EDU.VN today to access comprehensive comparisons, in-depth analyses, and expert insights. Let us help you find the perfect membrane to meet your needs!
Contact Information:
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
WhatsApp: +1 (626) 555-9090
Website: COMPARE.EDU.VN
This article provides a thorough comparison of different ion-exchange membranes, focusing on their relative dryness and how it affects their performance. For more detailed information and comparisons, visit compare.edu.vn.
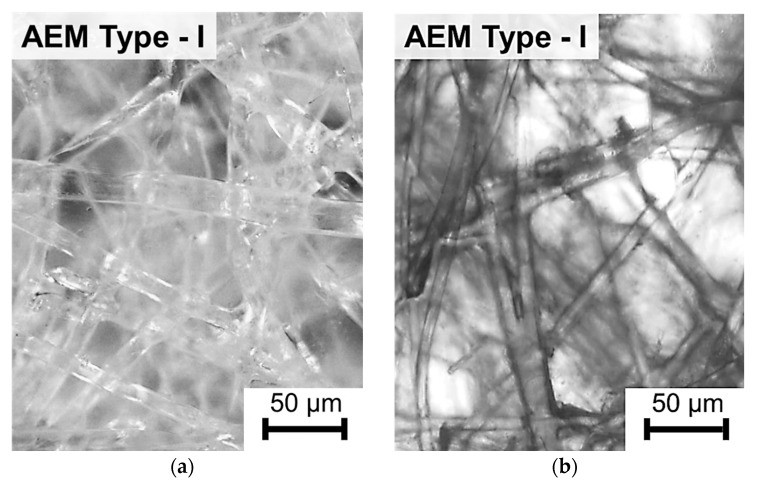 Optical images of the swollen AEM Type-I membrane showing high molecular weight dye penetration at the interface between fibers and ion-exchange material, indicating the presence of macropores
Optical images of the swollen AEM Type-I membrane showing high molecular weight dye penetration at the interface between fibers and ion-exchange material, indicating the presence of macropores
