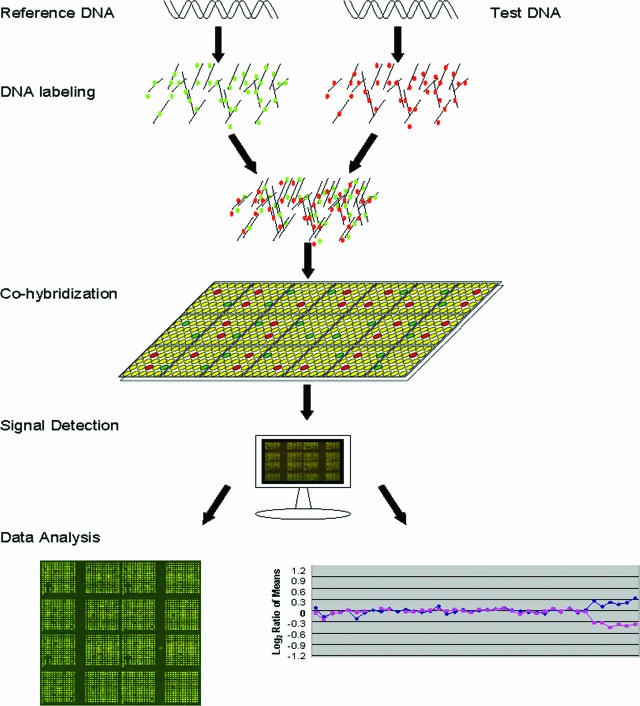
Comparative genomic hybridization (CGH), particularly array CGH, is a powerful molecular cytogenetic technique used to detect and analyze DNA copy number variations (CNVs) across the genome. This technique compares the genetic material of a test sample to a reference sample, identifying gains or losses of DNA segments. Let’s delve into the applications of this revolutionary technology.
How Does Comparative Genomic Hybridization Work?
Array CGH involves labeling DNA from a test and a reference sample with different fluorescent dyes. These labeled samples are then competitively hybridized to an array containing thousands of known DNA fragments (e.g., BAC clones). The relative fluorescence intensities of the two dyes at each spot on the array indicate the copy number ratio between the test and reference genomes at that specific location. A higher intensity of the test sample dye signifies a gain (duplication or amplification), while a higher intensity of the reference sample dye indicates a loss (deletion) in the test sample.
Figure: Schematic representation of array CGH. Differentially labeled test and reference DNA are co-hybridized to a microarray. Fluorescence ratios reveal copy number variations.
Research Applications of CGH
CGH has revolutionized genetic research, providing insights into various biological processes:
Gene Discovery: High-resolution array CGH enables the identification of specific genes associated with genetic disorders. By pinpointing regions of chromosomal gain or loss in affected individuals, researchers can narrow down the search for candidate genes. For example, CGH played a crucial role in identifying the CHD7 gene responsible for CHARGE syndrome.
Cancer Research: CGH is instrumental in characterizing the complex genomic alterations that drive cancer development and progression. It allows for the identification of characteristic CNV patterns, or “signatures,” in different cancer types, leading to a better understanding of tumor biology, potential therapeutic targets, and prognostic markers.
Epigenetic Studies: CGH can be adapted to study epigenetic modifications, such as DNA methylation, which play a role in gene regulation and disease.
Diagnostic Applications of CGH
The transition of CGH from research to diagnostics has significantly impacted clinical cytogenetics:
Developmental Disorders: Array CGH is increasingly used to diagnose individuals with unexplained developmental delays, intellectual disabilities, autism spectrum disorders, and multiple congenital anomalies. It can detect submicroscopic chromosomal imbalances that are often missed by traditional karyotyping.
Figure: Array CGH results illustrating a deletion on chromosome 10.
Prenatal Diagnosis: CGH can be applied to prenatal samples to detect chromosomal abnormalities in developing fetuses.
Cancer Diagnostics: In oncology, CGH aids in classifying tumors, predicting prognosis, and guiding treatment decisions.
Targeted vs. Whole-Genome CGH
While whole-genome arrays offer comprehensive coverage, targeted arrays focus on specific regions of clinical significance. Targeted arrays are often preferred in diagnostic settings as they provide clinically relevant information, reduce the complexity of data interpretation, and minimize the detection of benign CNVs.
Conclusion
Comparative genomic hybridization, specifically array CGH, is a versatile tool with broad applications in research and diagnostics. Its ability to detect genome-wide CNVs has significantly advanced our understanding of genetic diseases and cancer. In the clinical setting, array CGH is becoming the gold standard for identifying chromosomal abnormalities, leading to improved diagnosis, genetic counseling, and patient management. As technology continues to evolve, CGH will undoubtedly play an even greater role in unraveling the complexities of the human genome and its impact on health and disease.