Distilled water is known for its purity, making it a popular choice across various applications. But what other types of water are comparable to distilled water in terms of purity and uses? This article explores the similarities and differences between distilled water and deionized water, highlighting their respective pros and cons.
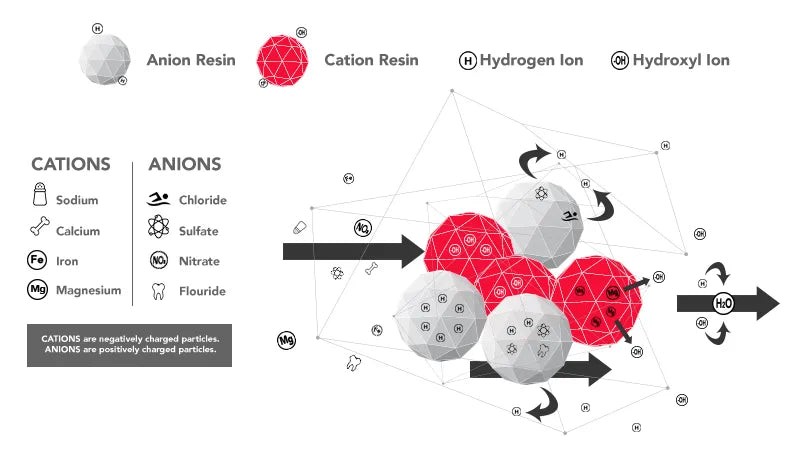 Deionization Chart, H2o Atoms, Cations, Anions, Hydrogen Ion, Hydroxyl Ion, Diagram of how Deionization works on an atomic level
Deionization Chart, H2o Atoms, Cations, Anions, Hydrogen Ion, Hydroxyl Ion, Diagram of how Deionization works on an atomic level
Both distilled and deionized water are considered extremely pure, but they achieve this purity through different processes. Understanding these differences is crucial in determining which type of water best suits your specific needs. Deionized (DI) water undergoes a process to remove all ions, primarily dissolved mineral salts. In contrast, distilled water is purified by boiling and collecting the condensed steam, leaving behind most impurities due to their higher boiling points.
Distillation, a time-tested method for water purification, involves heating filtered water to its boiling point. As the water transforms into steam, it’s collected in a sterile container. Upon condensation, the water returns to its liquid state, leaving behind contaminants with higher boiling points. This process results in highly purified water. Some processes even employ double or triple distillation for enhanced purity.
Water often contains organic materials and inorganic minerals as common impurities. Organic impurities are typically removed through various filtration methods such as physical filters, carbon filters, and reverse osmosis (RO) membranes. Following this pre-treatment, the water passes through a deionization system comprised of cation and anion resins. These resins attract and exchange positive and negative ions with H+ and OH- ions respectively. The resulting combination of H+ and OH- forms H2O, further purifying the water. The combined use of filtration and DI resins can effectively eliminate almost all contaminants.
While both methods produce highly purified water, deionized water may contain trace amounts of organic contaminants that are not removed by the ion exchange process. Distilled water, on the other hand, is generally free of both organic and inorganic impurities. However, the distillation process is typically more energy-intensive and costly than deionization. The choice between distilled and deionized water often depends on the specific application and the required level of purity. For some applications, the trace organics in deionized water may be acceptable, while others may require the higher purity of distilled water. Ultimately, considering both cost and efficiency is key when deciding between these two types of purified water.
