Abstract
Background
Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine has gained approval for the prevention of smallpox and monkeypox. However, the duration of its protective immunity and the effectiveness of booster doses have not been fully elucidated. This study aims to address these critical aspects of MVA-BN vaccination.
Methods
In this study, individuals who had never received a smallpox vaccine were randomly assigned to one of three groups: a single dose of MVA-BN (1×MVA, n = 181), two doses of MVA-BN (2×MVA, n = 183), or a placebo (n = 181). Additionally, participants with a history of smallpox vaccination were given a single MVA-BN booster (HSPX, n = 200). Two years later, a subset of the initially unvaccinated groups (approximately 75 individuals from each group) received an MVA-BN booster to assess long-term immune memory.
Results
The study revealed a significant increase in neutralizing antibody (nAb) geometric mean titers (GMTs) in the initially unvaccinated groups, rising from a baseline of 1.1 in both groups to 7.2 and 7.5 at week 4 for the 1×MVA and 2×MVA groups, respectively. Following the second vaccination in the 2×MVA group, nAb GMTs further increased to 45.6 at week 6. In the HSPX group, a rapid surge in nAb GMT was observed, from 21.6 at baseline to 175.1 at week 2 post-booster. After two years, nAb GMTs declined to 1.1, 1.3, and 10.3 in the 1×MVA, 2×MVA, and HSPX groups, respectively. Upon administration of a booster in the previously unvaccinated groups, a swift increase in nAb GMTs was observed within two weeks, reaching 80.7 (1×MVA) and 125.3 (2×MVA), levels exceeding those achieved after primary vaccination and comparable to the boosted HSPX group. Six months post-boosting, GMTs were measured at 25.6 (1×MVA) and 49.3 (2×MVA). Importantly, no safety concerns were identified throughout the study.
Conclusions
The observed anamnestic responses to booster doses, despite the decline in nAb titers over time, strongly suggest the presence of robust and durable immunological memory following primary MVA-BN vaccination. This indicates that MVA-BN offers comparable long-term immune protection, similar to what is expected from established vaccines against viral diseases.
Clinical Trials Registration. NCT00316524 and NCT00686582.
Keywords: monkeypox, booster, memory response, orthopoxvirus, recall response, revaccination, vaccinia experienced
Introduction
The global eradication of smallpox, achieved through widespread vaccination campaigns using replicating vaccinia-based vaccines, led to the discontinuation of routine smallpox vaccination in the 1970s. This cessation, while a triumph of public health, has resulted in a growing global population susceptible to variola virus, the causative agent of smallpox, and other orthopoxviruses, including the emergent threat of monkeypox. These traditional smallpox vaccines, while effective, were associated with rare but potentially serious adverse events, especially in vulnerable populations [10–12]. The risk of smallpox re-emergence, whether from laboratory accidents, occupational exposure [2], or bioterrorism [3], coupled with the increasing incidence and geographic spread of monkeypox [6–9], underscores the critical need for safe and effective orthopoxvirus vaccines and a deeper understanding of vaccine-induced immunity.
Monkeypox, initially confined to regions of Africa, has shown a concerning increase in human cases and international spread. Outbreaks in non-endemic countries, particularly since 2018 and notably the widespread outbreak starting in May 2022, highlight the vulnerability of populations lacking prior smallpox vaccination-derived immunity [8, 9]. This situation emphasizes the urgency to comprehend the immunogenicity and long-term protection offered by modern vaccines, especially in the context of evolving vaccination strategies and supply considerations.
The Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine represents a significant advancement in orthopoxvirus prevention. Developed using a highly attenuated MVA virus, MVA-BN is a non-replicating vaccine designed to mitigate the reactogenicity concerns associated with traditional replicating vaccinia vaccines [17–19]. MVA-BN has demonstrated immunogenicity comparable to traditional smallpox vaccines [18–20], while exhibiting a favorable safety profile in diverse populations, including healthy adults and immunocompromised individuals [21–34]. These attributes led to the approval of MVA-BN, marketed as Jynneos (US), Imvanex (EU, UK), and Imvamune (Canada), for the prevention of both smallpox and monkeypox.
This study investigates the duration and nature of immunological memory induced by MVA-BN vaccination in both individuals without prior smallpox vaccination and those vaccinated decades ago with live-replicating vaccinia vaccines. The findings are crucial for formulating optimal vaccination strategies, particularly in outbreak scenarios with limited vaccine supply, and for protecting individuals at occupational risk of orthopoxvirus exposure, such as healthcare workers and laboratory personnel. By evaluating the immune response and memory persistence, this research aims to define how MVA-BN compares to the established benchmarks of traditional smallpox vaccines in providing long-term protection against orthopoxviruses.
METHODS
Study Design
Two phase 2 clinical trials were conducted between 2006 and 2009 at a European site to evaluate the safety, immunogenicity, and booster response of MVA-BN in healthy adults, both with and without prior smallpox vaccination.
The initial study (NCT00316524) was a partially randomized, double-blind, placebo-controlled, non-inferiority trial. Participants with no prior smallpox vaccination history were randomized (1:1:1) to receive either a single dose of MVA-BN followed by a placebo dose four weeks later (1×MVA), two doses of MVA-BN four weeks apart (2×MVA), or two doses of Tris buffer placebo (PBO). Participants with a documented history of prior smallpox vaccination (HSPX) received a single booster dose of MVA-BN. The primary goal was to compare humoral immune responses between the HSPX and 2×MVA groups, assessing if a single MVA-BN booster in previously vaccinated individuals could elicit an immune response comparable to two primary MVA-BN vaccinations in unvaccinated individuals. Secondary objectives included comparing immune response kinetics across all groups. The safety results from the initial study have been previously published [25].
The follow-up study (NCT00686582) was an open-label phase 2 trial designed to assess the persistence of immunogenicity in the 1×MVA, 2×MVA, and HSPX groups two years after their last dose in the initial study. Furthermore, a subset of participants from the initially unvaccinated groups (1×MVA BD and 2×MVA BD groups) received an MVA-BN booster dose (BD) to evaluate post-booster immunogenicity and safety.
Participants
The studies were conducted in accordance with the Declaration of Helsinki and all participants provided informed consent. Eligibility criteria for the initial study included healthy men and non-pregnant women aged 18 to 55 years with normal laboratory values and agreement to use contraception. For the 1×MVA, 2×MVA, and PBO groups, participants had no known prior smallpox vaccination and no visible vaccinia scar. The HSPX group consisted of participants with documented prior smallpox vaccination or a typical vaccinia scar. Detailed eligibility criteria are available in the Supplementary Material. Participants who completed the initial study were invited to participate in the follow-up study two years later to assess humoral response persistence. Consenting participants from the 1×MVA and 2×MVA groups received a booster dose and were monitored for immunogenicity over six months.
Vaccine
The MVA-BN vaccine is a highly attenuated, purified, live, non-replicating vaccine [17]. Both the MVA-BN vaccine and the Tris buffer placebo were manufactured by IDT Biologika GmbH (Germany) following Good Manufacturing Practice standards. MVA-BN was supplied as liquid frozen 0.5-mL aliquots containing ≥ 0.5 × 108 TCID50 MVA titer. Vaccine and placebo were stored at −20°C ± 5°C, protected from light, and administered subcutaneously in the upper arm using a 24- or 25-gauge needle.
Immunogenicity Assessments
Serum total and neutralizing antibody titers were measured using enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization test (PRNT). In the initial study, samples were collected at screening, weeks 2, 4, 6, and 8, and at 6 months post-vaccination. In the follow-up study, samples were collected at the 2-year mark (−2 to +3 months) from all available participants. Boosted participants also had samples drawn at 1, 2, and 4 weeks, and 6 months post-boosting. Detailed ELISA and PRNT methodologies are provided in the Supplementary Material.
Safety Assessments
Safety assessments for the initial study have been previously reported [25]. The follow-up study safety assessments focused on solicited local and systemic adverse events (AEs), unsolicited AEs, and serious adverse events (SAEs). Solicited AEs were predefined expected reactions, recorded by participants for 8 days post-booster using a memory aid (see Supplementary Material). Unsolicited AEs were reported at study visits. Causality for unsolicited AEs and SAEs was determined by the study investigator. Safety laboratory tests were conducted at screening and 2 weeks post-booster. Adverse events of special interest (AESIs), specifically cardiac events, were closely monitored with follow-up examinations and diagnostic testing until resolution.
Statistical Methods
Statistical analyses were performed using SAS 9.1. Safety and primary immunogenicity analyses were based on the full analysis set (all vaccinated participants). Immunogenicity was also analyzed for the per-protocol set (participants adhering to protocol conditions). Randomization lists were used in the initial study. Unsolicited AEs were coded using Medical Dictionary of Regulatory Activities terminology. Seroconversion definitions differed based on baseline serostatus (see Results section for details). Geometric mean titers (GMTs) were calculated using log10 transformations of titers. Antibody titers below the detection limit were assigned a value of 1.
RESULTS
Clinical Participant Population and Conduct of the Study
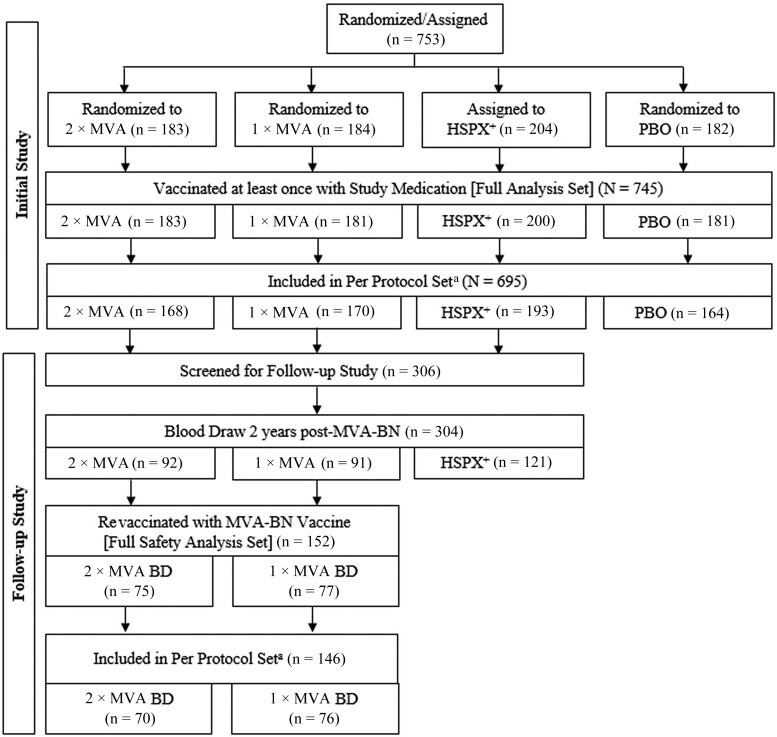
The initial study enrolled 753 participants, including 204 individuals with a history of smallpox vaccination (HSPX group) who received an MVA-BN booster. The remaining 549 participants without prior smallpox vaccination were randomized to the 1×MVA, 2×MVA, or PBO groups (Figure 1). Eight participants not meeting eligibility criteria were excluded. Primary MVA-BN vaccination was administered to 181 participants in the 1×MVA group and 183 in the 2×MVA group; 181 received placebo. 200 HSPX participants received the MVA-BN booster. In total, 745 participants were included in the full analysis set.
Two years later, 304 participants (91 from 1×MVA, 92 from 2×MVA, 121 from HSPX) provided blood samples for antibody persistence assessment. 77 and 75 participants initially in the 1×MVA and 2×MVA groups, respectively, received an MVA-BN booster.
Baseline demographics were similar across treatment groups (Table 1). Slightly over half were female (53.0% to 61.9%), and predominantly white (97.2% to 99.0%). The HSPX group was older (mean age 41.5 years) compared to the other groups (25.3 and 26.0 years), reflecting their vaccination history prior to smallpox eradication in 1980. Concomitant medication use at baseline was reported in 64.4%, mainly over-the-counter pain relievers and contraceptives. The follow-up study population exhibited similar baseline characteristics.
Immunogenicity
Early Responses to Vaccination, Initial Study
In the 1×MVA group, neutralizing antibody (nAb) GMTs rapidly increased from baseline (1.1) to week 4 (7.2) (Figure 2A). A similar increase was seen after the first vaccination in the 2×MVA group, reaching 7.5 at week 4. Following the second MVA-BN vaccination in the 2×MVA group, nAb GMTs peaked at week 6 (45.6), almost tenfold higher than two weeks after the first dose. GMTs remained elevated at week 8.
In the HSPX group, nAb GMTs surged from baseline (21.6) to week 2 (175.1), approximately four times greater than the 2×MVA group at week 6. nAb GMTs remained high four weeks post-booster (144.3) (Figure 2A).
Approximately half of participants in the 1×MVA and 2×MVA groups seroconverted for nAb at week 2, increasing to 62.1% and 56.7% by week 4, respectively (Figure 3A). The second vaccination in the 2×MVA group further increased seroconversion to 89.2% by week 6.
The HSPX group showed 78.5% seroconversion at week 2, despite a wide range of baseline neutralizing antibodies.
Total antibody trends mirrored nAb trends (Figure 2C and Figure 3B). Peak total antibody titers were comparable between the HSPX booster group and the 2×MVA primary vaccination group. As expected, the PBO group showed negligible antibody responses.
Immunogenicity at 6 Months and 2 Years
At 6 months (week 30), nAb GMTs remained higher than baseline in the HSPX group (106.5). Sustained, albeit lower, nAb levels above baseline were also observed in the 1×MVA (1.9) and 2×MVA (7.2) groups (Figure 2A). nAb seroconversion rates at 6 months were 23.6% (1×MVA) and 65.2% (2×MVA) (Figure 3A). By 2 years, nAb GMTs had returned to near baseline levels (Figure 2A).
Total antibody GMTs at 2 years remained above baseline in all groups, most notably in the HSPX group (134.7 vs 38.8 baseline), and more modestly in the 2×MVA (23.3 vs 1.4 baseline) and 1×MVA (6.2 vs 1.3 baseline) groups (Figure 2*C). Sustained total antibody seroconversion from 6 months to 2 years was observed in the 1×MVA (37.9% and 42.9%) and 2×MVA (73.0% and 71.7%) groups (Figure 3B*).
Booster Immunogenicity, Follow-up Study
Revaccination with a single MVA-BN booster 2 years post-initial study induced rapid nAb GMT increases within one week, peaking at 2 weeks (week 110) in both 1×MVA BD and 2×MVA BD groups (80.7 and 125.3, respectively; Figure 2B). These booster responses, similar in magnitude and kinetics between groups, surpassed peak responses after primary vaccination. Four weeks post-booster (week 112), nAb GMTs decreased by nearly half, with further decline by 6 months, although levels remained elevated compared to pre-booster and 6-month post-primary vaccination levels.
Nearly all participants showed rapid nAb seroconversion post-booster, peaking at 2 weeks (96.1% for 1×MVA BD and 98.7% for 2×MVA BD). Seroconversion rates remained high at 6 months (76.6% and 88.7%) (Figure 3A).
Total antibodies also showed rapid post-booster increases (Figure 2D and Figure 3B). Total antibody GMTs after booster were higher than after primary vaccination and nearly threefold higher in the 2×MVA BD group compared to the HSPX group in the initial study. Detailed GMT values are in Supplementary Table 1. Per-protocol set immunogenicity results were consistent with the full analysis set.
Overall Safety Assessment
Follow-up study safety results are presented herein, as initial study safety results are previously published [25]. Solicited local AEs were predominantly injection site erythema (82.2%) and pain (80.3%). Common systemic AEs were fatigue (32.2%), myalgia (23.7%), and headache (28.9%) (Table 2). Most solicited AEs were mild or moderate; grade 3 events (headache, myalgia, fatigue) occurred in one 1×MVA and three 2×MVA participants. No grade 4 events were reported.
Approximately half (51.3%) experienced unsolicited AEs, with 13.2% considered vaccine-related (Table 2). Injection site warmth was the most common related unsolicited AE (3.9%) (Table 3). One severe, possibly related unsolicited AE (pain in extremity) resolved the same day.
Two participants (1.3%) in the 2×MVA group experienced SAEs (gastroenteritis, concussion), neither related to vaccination. Five participants (3.3%) experienced AESIs (palpitations, chest pain), all mild/moderate and unlikely related to vaccination. No deaths or study discontinuations due to AEs occurred.
DISCUSSION
This study demonstrates that an MVA-BN vaccine booster dose elicits a strong immune response, regardless of prior smallpox vaccination status or the number of primary MVA-BN doses received. Two years post-primary vaccination in previously unvaccinated individuals, a booster dose induced a rapid increase in nAb titers, peaking at 2 weeks and remaining elevated at 6 months. A single MVA-BN booster in individuals vaccinated decades prior with older replicating smallpox vaccines also induced a similar rapid increase in nAb titers. The anamnestic nAb response magnitude was comparable across groups, though slightly lower in MVA-BN primed groups. Booster responses exceeding primary vaccination responses are consistent with prior observations for other smallpox vaccines [36].
These findings suggest that one or two doses of MVA-BN can establish B-cell immune memory comparable to older replicating smallpox vaccines, which historically provided long-term protection against orthopoxviruses, including monkeypox [6, 7]. While neutralizing antibodies in individuals vaccinated long ago were detectable decades later, a definitive protective threshold for smallpox or monkeypox remains undefined [37]. In this study, nAb levels in MVA-BN primed individuals had declined to near baseline at the time of boosting, yet a single booster dose induced robust anamnestic responses, mirroring the rapid kinetics seen in individuals boosted after receiving older replicating vaccines. This rapid recall response highlights the presence of durable B-cell memory, suggesting protection even with low or absent circulating nAbs, especially considering the incubation periods for smallpox and monkeypox (over one week) [38, 39].
Animal studies further support sustained protection with MVA-BN. Nonhuman primates challenged with monkeypox virus exhibited protection despite nAb titers declining to pre-vaccination levels after approximately 3 years. Upon viral exposure, antibody titers rapidly increased, and the animals were protected from disease [41, 42]. This suggests that even a single MVA-BN dose may offer long-term protection against future orthopoxvirus exposures.
This pattern of long-term immunological memory in the absence of sustained high nAb levels is also observed with vaccines for hepatitis B virus (HBV) and measles, mumps, and rubella (MMR) [43–46]. These vaccines demonstrate that robust memory responses can provide protection even when circulating antibody titers wane over time.
In this study, MVA-BN booster vaccination induced robust total antibody responses across all groups. Interestingly, total antibody titers post-booster were substantially higher in MVA-BN primed groups compared to those primed with older replicating vaccines. While the time difference between priming and boosting might influence this, emerging evidence suggests non-neutralizing antibodies may also contribute to protection against viral infections [47, 48]. These antibodies can activate effector pathways and target conserved viral proteins, potentially contributing to cross-protection against orthopoxviruses like monkeypox [41, 42].
Safety findings in the follow-up study were consistent with the initial study, with no new safety concerns identified. Continuous cardiac safety monitoring throughout MVA-BN clinical development, prompted by myopericarditis reports with replicating smallpox vaccines [10, 12, 49, 50], has not revealed any cardiac safety signals or cases of myo-/pericarditis with MVA-BN [23, 25, 26]. The observed AESIs in this study were deemed unrelated to vaccination, further supporting the favorable safety profile of MVA-BN. These immunological and safety findings are expected to be consistent with the freeze-dried formulation of MVA-BN, which has shown comparable immunogenicity and safety.
Study limitations include the single European site and limited racial/ethnic diversity. However, other MVA-BN studies with more diverse populations have not shown racial differences in immunogenicity or safety [19]. Another limitation is the use of vaccinia-based antigens in immune assays, rather than monkeypox-specific antigens, which is consistent with the evaluation of other smallpox vaccines known to confer protection against smallpox.
CONCLUSION
Primary immunization with one or two doses of MVA-BN induces durable immune memory. Boosting two years later results in a rapid and substantial anamnestic response, comparable to boosting after immunization with older replicating smallpox vaccines. This robust memory response underscores the potential of MVA-BN as a crucial tool in the ongoing efforts to control and prevent orthopoxvirus infections, offering a comparable and safe alternative to traditional vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online.
Supplementary Material
jiac455_Supplementary_Data
Click here for additional data file. (39.9KB, docx)
Contributor Information
Heiko Ilchmann, Harrison Clinical Research Deutschland, GmbH, Munich, Germany.
Nathaly Samy, Bavarian Nordic, GmbH, Martinsried, Germany.
Daniela Reichhardt, Bavarian Nordic, GmbH, Martinsried, Germany.
Darja Schmidt, Bavarian Nordic, GmbH, Martinsried, Germany.
Jacqueline D Powell, Bavarian Nordic, Inc, Durham, North Carolina, USA.
Thomas P H Meyer, Division of Infectious Diseases and Tropical Medicine, LMU University Hospital, Munich, Germany.
Günter Silbernagl, Bavarian Nordic, GmbH, Martinsried, Germany.
Rick Nichols, Public Health Vaccines, LLC, Cambridge, Massachusetts, USA.
Heinz Weidenthaler, Bavarian Nordic, GmbH, Martinsried, Germany.
Laurence De Moerlooze, Bavarian Nordic, AG, Zug, Switzerland.
Liddy Chen, Bavarian Nordic, Inc, Durham, North Carolina, USA.
Paul Chaplin, Bavarian Nordic, A/S, Kvistgård, Denmark.
Notes
Author contributions. H. I., N. S., D. R., D. S., R. N., J. D. P., T. P. H. M., G. S., L. D. M., L. C., H. W., and P. C. contributed to the manuscript.
Acknowledgments. The investigators thank all study participants and acknowledge the contributions of Frank von Sonnenburg and Stephan de la Motte.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number N01-AI40072); and sponsored by Bavarian Nordic.
References
Associated Data
Supplementary Materials
jiac455_Supplementary_Data
Click here for additional data file. (39.9KB, docx)