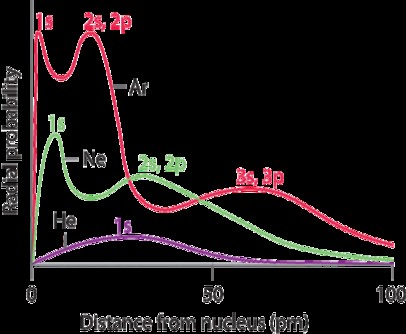
Ionic radii comparison can be tricky. This detailed guide on COMPARE.EDU.VN simplifies the process. Understand how to effectively compare ionic radii and make informed decisions. This article also provides crucial information and insights into periodic trends and isoelectronic series, making it easier than ever to compare.
Introduction to Ionic Radii and Their Significance
Ionic radii play a crucial role in determining the properties and behavior of chemical compounds. COMPARE.EDU.VN offers a comprehensive look at how to compare these radii, providing a foundation for understanding chemical interactions and material properties. Understanding the periodic trends and the impact of effective nuclear charge is essential for accurate comparisons. This article explores the intricacies of ionic sizes and provides a clear methodology for comparing different ions, crucial for anyone studying chemistry or materials science. We will cover effective nuclear charge, isoelectronic series and factors influencing Ionic Radii.
1. Defining Atomic and Ionic Radii
1.1. Understanding Atomic Radii
Atomic radii are a measure of the size of an atom, but unlike a solid sphere, atoms don’t have a definite boundary. The electron cloud fades gradually, making it tough to pinpoint an exact edge. However, knowing the atomic radius helps predict how atoms interact in molecules.
1.2. Measuring Atomic Radii
Chemists use various methods to define atomic radii:
- Covalent Atomic Radius ((r_{cov})): Half the distance between the nuclei of two identical atoms joined by a covalent bond.
- Metallic Atomic Radius ((r_{met})): Half the distance between the nuclei of two adjacent atoms in a solid metal.
- Van der Waals Atomic Radius ((r_{vdW})): Half the internuclear distance between two nonbonded atoms in a solid.
1.3. Defining Ionic Radii
Ionic radii refer to the size of ions, which are atoms that have gained or lost electrons. Cations (positive ions) are smaller than their parent atoms, while anions (negative ions) are larger.
2. Factors Influencing Ionic Radii
2.1. Effective Nuclear Charge
The effective nuclear charge ((Z{eff})) is the net positive charge experienced by an electron in an atom. It’s influenced by the actual nuclear charge (number of protons) and the shielding effect of inner electrons. A higher (Z{eff}) pulls the electrons closer to the nucleus, decreasing the atomic or ionic radius.
2.2. Electron Shielding
Electron shielding occurs when inner electrons reduce the attractive force between the nucleus and outer electrons. Electrons in the same principal shell shield each other less effectively than those in inner shells.
2.3. Principal Quantum Number (n)
The principal quantum number (n) indicates the energy level and size of an electron orbital. As n increases, the orbital size also increases, leading to larger atomic and ionic radii, provided the nuclear charge remains constant.
3. Periodic Trends in Atomic Radii
3.1. Trends Across a Period
Atomic radii generally decrease from left to right across a period in the periodic table. This is because the nuclear charge increases, while electrons are added to the same principal shell. The increasing nuclear charge pulls the electrons closer, reducing the atomic size.
3.2. Trends Down a Group
Atomic radii increase from top to bottom down a group. As you move down, electrons are added to higher energy levels (larger n), and inner electrons effectively shield the outer electrons from the full nuclear charge.
4. Comparing Ionic Radii: Key Considerations
4.1. Cations vs. Anions
Cations are always smaller than their parent atoms because the removal of electrons reduces electron-electron repulsion and increases the effective nuclear charge. Conversely, anions are always larger than their parent atoms due to increased electron-electron repulsion and decreased effective nuclear charge upon adding electrons.
4.2. Isoelectronic Series
An isoelectronic series consists of ions that have the same number of electrons but different nuclear charges. Within an isoelectronic series, ionic radius decreases as the nuclear charge increases.
4.3. Oxidation State
The oxidation state of an ion significantly affects its size. Higher positive charges result in smaller ionic radii, while higher negative charges lead to larger ionic radii. For example, (Fe^{3+}) is smaller than (Fe^{2+}).
5. How to Compare Ionic Radii Effectively
5.1. Step-by-Step Guide
- Determine the Electronic Configuration: Identify the number of electrons each ion possesses.
- Assess the Nuclear Charge: Determine the number of protons in each ion’s nucleus.
- Consider Isoelectronic Series: If ions are isoelectronic, compare their nuclear charges.
- Analyze Oxidation States: Account for the charge of the ion; higher positive charges decrease radii.
- Refer to Periodic Trends: Use the periodic table to estimate relative sizes based on group and period positions.
- Consult Reliable Sources: Utilize databases or textbooks for specific ionic radii values.
5.2. Using Tables and Charts
Referencing tables and charts of ionic radii can help compare ions effectively.
5.3. Examples of Ionic Radii Comparisons
5.3.1. Comparing (Na^+) and (Mg^{2+})
Both (Na^+) and (Mg^{2+}) are isoelectronic, each having 10 electrons. However, (Mg^{2+}) has a greater nuclear charge (+12) than (Na^+) (+11). Therefore, (Mg^{2+}) is smaller than (Na^+).
5.3.2. Comparing (O^{2-}) and (F^-)
Both (O^{2-}) and (F^-) have 10 electrons. (F^-) has a nuclear charge of +9, while (O^{2-}) has +8. As a result, (F^-) is smaller than (O^{2-}).
5.3.3. Comparing (Cl^-) and (K^+)
Both (Cl^-) and (K^+) are isoelectronic, each possessing 18 electrons. (K^+) has a greater nuclear charge (+19) compared to (Cl^-) (+17). Therefore, (K^+) is smaller than (Cl^-).
6. Ionic Radii and Chemical Properties
6.1. Lattice Energy
Ionic radii play a pivotal role in determining the lattice energy of ionic compounds. Lattice energy is the energy required to separate one mole of an ionic compound into its gaseous ions. Smaller ions with higher charges lead to greater lattice energies.
6.2. Coordination Number
The coordination number, which is the number of ions surrounding a central ion in a crystal lattice, is influenced by ionic radii. The relative sizes of cations and anions affect the arrangement and stability of the crystal structure.
6.3. Solubility
Ionic radii also impact the solubility of ionic compounds. Smaller ions with higher charges tend to form stronger electrostatic interactions, which can decrease solubility in polar solvents.
7. Common Pitfalls in Comparing Ionic Radii
7.1. Ignoring Oxidation State
Failing to consider the oxidation state can lead to incorrect conclusions. Always account for the charge of the ion when comparing sizes.
7.2. Neglecting Isoelectronic Nature
When comparing ions, determine if they are isoelectronic. This simplifies the comparison, as nuclear charge becomes the primary factor.
7.3. Overlooking Periodic Trends
Always consider the positions of elements in the periodic table. This provides a baseline understanding of relative sizes.
8. Advanced Concepts in Ionic Radii
8.1. Polarization Effects
Ions can polarize the electron cloud of neighboring ions, which affects their effective sizes. Smaller, highly charged cations are more polarizing.
8.2. Non-Spherical Ions
Some ions deviate from perfect spherical shapes due to uneven electron distributions. This can complicate size comparisons.
8.3. Hydration Radii
When ions dissolve in water, they become hydrated. Hydration radii, which include the water molecules surrounding the ion, can differ significantly from bare ionic radii.
9. Case Studies: Applications of Ionic Radii
9.1. Mineralogy
Ionic radii are crucial in mineralogy for understanding the composition and structure of minerals. They influence the substitution of ions in crystal lattices.
9.2. Materials Science
In materials science, ionic radii help design and predict the properties of new materials, such as ceramics and semiconductors.
9.3. Biochemistry
Ionic radii play a role in enzyme-substrate interactions and ion channel selectivity in biological systems.
10. Tools and Resources for Comparing Ionic Radii
10.1. Online Databases
Several online databases provide ionic radii values:
- WebElements
- PubChem
- Crystal Structures Database
10.2. Textbooks and Academic Journals
Consult chemistry textbooks and academic journals for detailed information on ionic radii and related concepts.
10.3. Software and Modeling Tools
Computational chemistry software can model and predict ionic radii based on electronic structure calculations.
11. Real-World Applications of Ionic Radii
11.1. Battery Technology
Ionic radii are essential in designing and improving battery performance. Lithium-ion batteries rely on the small size of lithium ions to facilitate their movement within the battery.
11.2. Catalysis
In catalysis, ionic radii influence the activity and selectivity of catalysts. The size and charge of ions in catalytic materials can affect their ability to bind and activate reactants.
11.3. Environmental Science
Ionic radii are used to study the behavior of ions in the environment, such as the mobility of heavy metals in soil and water.
12. The Future of Ionic Radii Research
12.1. New Measurement Techniques
Advancements in experimental techniques, such as X-ray diffraction and neutron diffraction, are providing more accurate ionic radii measurements.
12.2. Computational Chemistry
Computational chemistry methods are becoming increasingly sophisticated, allowing for more precise predictions of ionic radii and their effects on material properties.
12.3. Interdisciplinary Applications
The application of ionic radii concepts is expanding into interdisciplinary fields, such as nanosciences and biotechnology.
13. Conclusion: Mastering Ionic Radii Comparisons
Understanding How To Compare Ionic Radii is crucial for anyone studying chemistry, materials science, or related fields. By considering effective nuclear charge, electron shielding, isoelectronic series, and periodic trends, one can accurately predict and explain the properties of chemical compounds and materials. Use this guide from COMPARE.EDU.VN to enhance your understanding and decision-making process.
Ionic radii play a significant role in the properties and behavior of chemical compounds. Understanding how to compare these radii provides a solid foundation for interpreting chemical interactions and material characteristics. Keep in mind that effective nuclear charge and periodic trends are essential for accurate comparisons.
14. FAQs About Comparing Ionic Radii
14.1. What is the main factor affecting ionic radii?
The main factor affecting ionic radii is the effective nuclear charge, which is the net positive charge experienced by the outermost electrons.
14.2. How do you compare ionic radii in an isoelectronic series?
In an isoelectronic series, ionic radius decreases as the nuclear charge (number of protons) increases.
14.3. Are cations always smaller than anions?
Yes, cations are always smaller than their parent neutral atoms, and anions are always larger than their parent neutral atoms.
14.4. Why do ionic radii increase down a group?
Ionic radii increase down a group because electrons are added to higher energy levels, which are further from the nucleus.
14.5. How does oxidation state affect ionic radii?
Higher positive oxidation states lead to smaller ionic radii, while higher negative oxidation states result in larger ionic radii.
14.6. What is an isoelectronic series?
An isoelectronic series is a group of ions or atoms that have the same number of electrons but different nuclear charges.
14.7. Can you predict ionic radii accurately?
You can estimate ionic radii based on periodic trends and general principles, but consulting reliable sources is essential for accurate values.
14.8. What is the role of ionic radii in lattice energy?
Ionic radii influence the lattice energy of ionic compounds, with smaller ions generally leading to higher lattice energies.
14.9. How do hydration radii differ from bare ionic radii?
Hydration radii include the water molecules surrounding an ion in solution, while bare ionic radii refer to the size of the ion without any surrounding water molecules.
14.10. Where can I find reliable values for ionic radii?
You can find reliable values for ionic radii in online databases, chemistry textbooks, and academic journals.
15. Further Resources on COMPARE.EDU.VN
15.1. Related Articles
Explore COMPARE.EDU.VN for more in-depth articles on atomic structure, periodic trends, and chemical bonding.
15.2. Expert Comparisons
Check out our expert comparisons of different chemical elements and compounds, providing detailed analysis and insights.
15.3. Community Forums
Join our community forums to discuss ionic radii and other chemistry topics with experts and fellow learners.
16. Take the Next Step with COMPARE.EDU.VN
Ready to make informed decisions about chemical compounds and their properties? Visit COMPARE.EDU.VN today to access our comprehensive comparison tools and resources. Whether you’re a student, researcher, or industry professional, COMPARE.EDU.VN is your trusted source for objective and detailed comparisons.
Still struggling to compare ionic radii effectively? Don’t worry COMPARE.EDU.VN can help. Explore our resources for comprehensive and objective comparisons that simplify complex decisions. Visit COMPARE.EDU.VN today.
Address: 333 Comparison Plaza, Choice City, CA 90210, United States.
Whatsapp: +1 (626) 555-9090.
Website: compare.edu.vn