Electronegativity measures an atom’s ability to attract electrons in a chemical bond. Understanding electronegativity is crucial for predicting bond type and molecular properties. This article explains how to compare electronegativity and discusses factors influencing it.
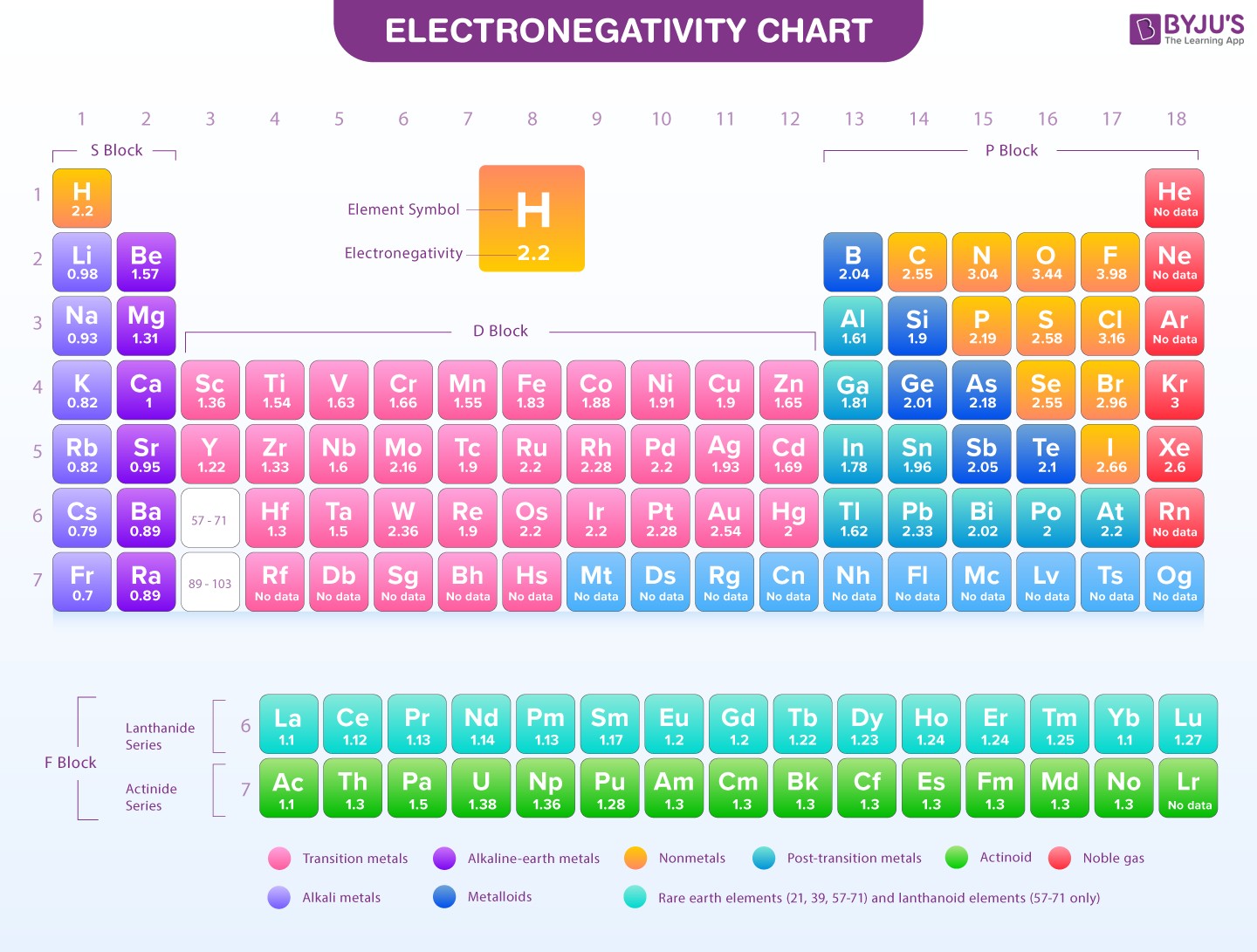 Electronegativity Chart
Electronegativity Chart
Understanding Electronegativity Trends in the Periodic Table
Electronegativity values are not absolute but relative, often based on the Pauling scale where fluorine (F), the most electronegative element, is assigned a value of 4.0. Cesium (Cs) and Francium (Fr) are among the least electronegative, with values around 0.7. Generally, electronegativity increases across a period (from left to right) and decreases down a group (from top to bottom) in the periodic table. This trend is due to changes in atomic size and nuclear charge.
Factors Affecting Electronegativity
Several factors influence an atom’s electronegativity:
1. Atomic Size
Smaller atoms have higher electronegativity. The closer valence electrons are to the positively charged nucleus, the stronger the attraction and thus the higher the electronegativity. Larger atoms with more electron shells shield the valence electrons from the nucleus, reducing electronegativity.
2. Nuclear Charge
A higher nuclear charge (more protons) increases electronegativity. A stronger positive charge in the nucleus exerts a greater pull on electrons, even accounting for shielding from inner electron shells.
3. Substituent Effects
The electronegativity of an atom can be influenced by the atoms it’s bonded to. Electron-withdrawing groups attached to an atom can increase its electronegativity, while electron-donating groups can decrease it. For example, a carbon atom in CF3I is more electronegative than a carbon atom in CH3I due to the strong electron-withdrawing effect of fluorine.
Electronegativity and Chemical Bonding
Electronegativity differences between atoms dictate the nature of chemical bonds:
-
Nonpolar Covalent Bonds: Atoms with similar electronegativities share electrons equally, forming nonpolar covalent bonds (e.g., H2, Cl2).
-
Polar Covalent Bonds: When electronegativity differs moderately, the more electronegative atom attracts electrons more strongly, creating partial positive (δ+) and negative (δ-) charges and forming a polar covalent bond (e.g., H2O).
-
Ionic Bonds: Large electronegativity differences lead to the more electronegative atom essentially stealing electrons from the other, forming ions and resulting in an ionic bond (e.g., NaCl). The electronegativity difference threshold for predominantly ionic character is generally considered to be around 1.7.
Using Electronegativity to Predict Bond Polarity
Comparing the electronegativity values of two bonded atoms helps determine bond polarity:
-
Find Electronegativity Values: Consult a periodic table or electronegativity chart to find the electronegativity values for each element.
-
Calculate the Difference: Subtract the smaller electronegativity value from the larger one to find the difference.
-
Interpret the Difference:
- 0 – 0.4: Nonpolar covalent bond
- 0.4 – 1.7: Polar covalent bond
- > 1.7: Ionic bond
Conclusion
Electronegativity is a fundamental concept in chemistry. By understanding how to compare electronegativity values and the factors that influence them, we can predict bond types, molecular polarity, and other important chemical properties. Utilizing the periodic trends and considering the factors discussed above allows for effective comparison of electronegativity between elements.