DNA, the blueprint of life, is remarkably complex yet incredibly tiny. But just How Small Is Dna Compared To An Atom, the fundamental building block of matter? This exploration delves into the scale of DNA, comparing it to the size of atoms and other microscopic entities.
Visualizing the Microscopic World: Cells and Beyond
The human eye, unaided, can perceive objects as small as 0.1 mm. This allows us to see certain large single-celled organisms like amoebas and paramecia. A light microscope enhances our vision, revealing the intricate structures within cells like the nucleus and mitochondria. However, light microscopes are limited by the wavelength of light, restricting their resolution to around 500 nm.
Fig 1: Diagram of a light microscope. It uses a system of lenses to magnify an image.
To visualize objects smaller than 500 nm, such as viruses and molecules, we require the power of an electron microscope. Electron microscopes utilize a beam of electrons, with a much smaller wavelength than light, enabling them to resolve even individual atoms.
Deoxyribonucleic Acid: The Building Blocks of Life
Fig 2: Structure of a nucleotide, the building block of DNA.
DNA, or deoxyribonucleic acid, comprises nucleotides, each containing a sugar (deoxyribose), a phosphate group, and a nitrogenous base (like adenine). While often simply labeled “adenine,” a nucleotide is a more complex structure. The term “deoxyadenosine monophosphate” provides a more accurate description.
The Astonishing Size Difference: DNA, Chromosomes, and Atoms
The size difference between a chromosome and a sperm head might seem counterintuitive. However, the DNA within a sperm cell is incredibly condensed. Specialized proteins called protamines compact the DNA to about one-sixth the volume of a chromosome in a dividing cell. This dense packing allows the sperm to be an efficient delivery system for genetic material.
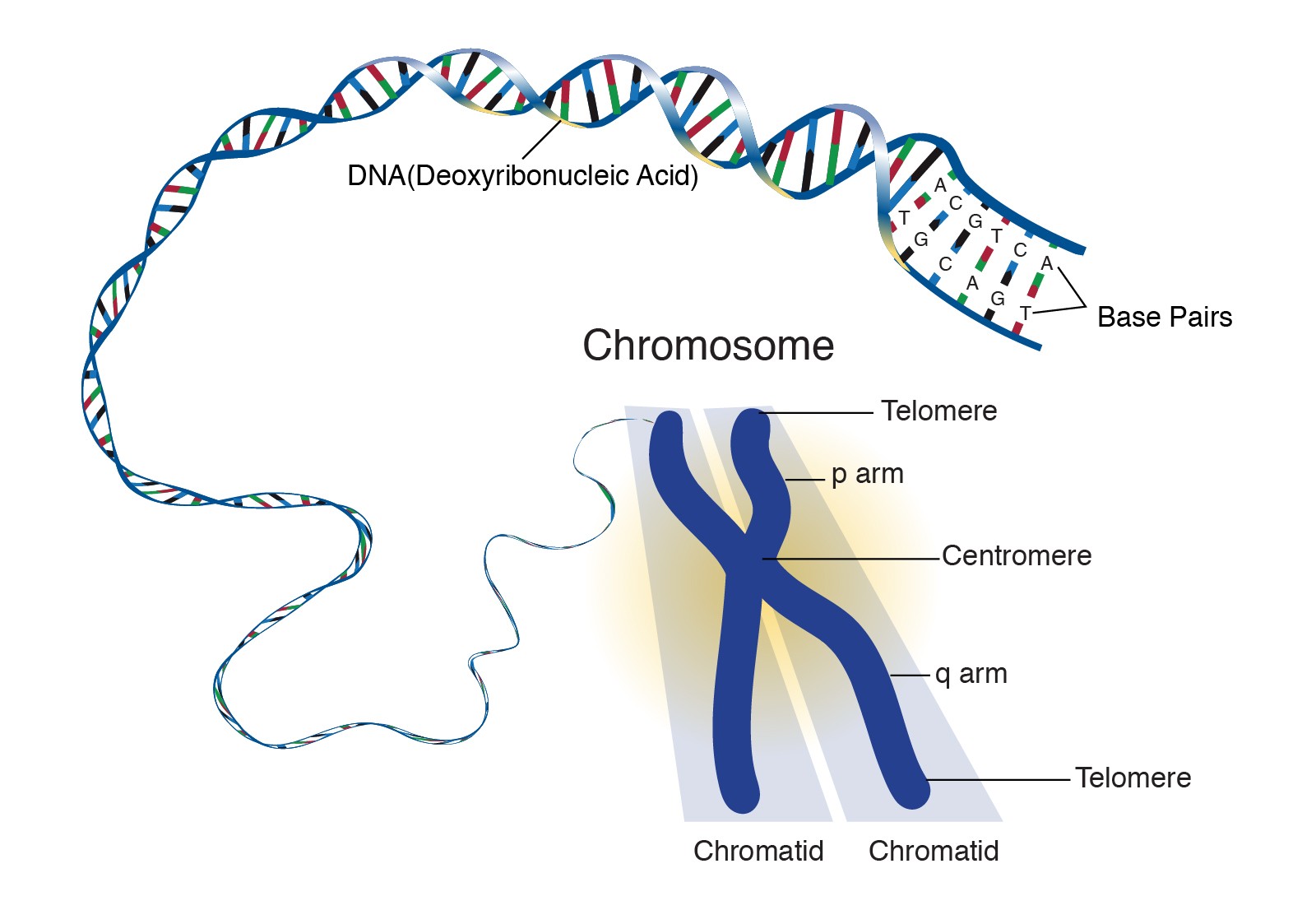 Chromosome
Chromosome
Fig 3: Illustration of a chromosome, showing the tightly packed DNA.
Zooming in further, we encounter the fundamental building blocks of all matter: atoms. An atom’s size is typically measured by its van der Waals radius, representing the distance between two atoms’ nuclei when they are not bonded. A carbon atom, for instance, has a van der Waals radius of approximately 0.17 nm.
Fig 4: Structure of a carbon atom, a fundamental building block of matter.
Compared to a carbon atom’s minuscule size (0.17 nm), the DNA double helix, with a diameter of about 2 nm, is significantly larger. However, DNA is still incredibly small relative to objects we encounter in our everyday lives.
Conclusion: A Matter of Scale
DNA, while significantly larger than an atom, remains astonishingly small. This dramatic difference in scale highlights the complexity of biological systems and the power of scientific tools like electron microscopes, which allow us to visualize the fundamental building blocks of life. The journey from visible cells to the invisible realm of atoms reveals a universe of intricate structures and underscores the vastness within the smallest of spaces.
