Hybrid orbitals are crucial for understanding molecular bonding and geometry. At COMPARE.EDU.VN, we explore how the energy levels of these hybrid orbitals compare to those of atomic orbitals, providing a clear understanding of their significance. This analysis will provide an explanation of valence bond theory, molecular shape and VSEPR theory along with linear combinations of atomic orbitals.
1. Introduction to Hybrid Orbitals
Hybrid orbitals are formed through a process called hybridization, where atomic orbitals mix to form new hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. This concept helps explain molecular geometry and bonding properties that simple atomic orbital overlap cannot. The understanding of hybridization is vital in predicting and explaining molecular structures, bond angles, and overall molecular behavior. The essence of this article delves into the relationship between atomic orbitals, hybrid orbitals, and molecular geometry which is easily accessible on COMPARE.EDU.VN.
1.1 What are Atomic Orbitals?
Atomic orbitals are mathematical functions that describe the wave-like behavior of an electron in an atom. These orbitals represent regions in space where there is a high probability of finding an electron. The principal quantum number (n) defines the energy level or shell of the electron, with higher values of n indicating higher energy levels and greater distances from the nucleus. Within each energy level, electrons occupy sublevels or subshells, which are designated by the azimuthal quantum number (l). The values of l range from 0 to n-1, with l = 0, 1, and 2 corresponding to s, p, and d orbitals, respectively.
Each type of orbital has a distinct shape:
- s orbitals: Spherical in shape, centered around the nucleus.
- p orbitals: Dumbbell-shaped, with two lobes oriented along the x, y, and z axes.
- d orbitals: More complex shapes, with most having four lobes.
The energy of an electron in an atomic orbital depends primarily on the principal quantum number n. For a given atom, the energy levels increase in the order s < p < d < f. Understanding the arrangement and energies of atomic orbitals is crucial for comprehending how atoms form chemical bonds and create molecules.
1.2 Why Hybridization is Necessary
Hybridization becomes necessary when the observed molecular geometries and bonding properties of molecules cannot be adequately explained by the direct overlap of atomic orbitals. One classic example is methane (CH4). Carbon has an electronic configuration of 1s²2s²2p². According to valence bond theory, carbon should form two bonds using its two unpaired 2p electrons, resulting in a CH2 molecule with a 90° bond angle. However, methane is a tetrahedral molecule with four equivalent C-H bonds and bond angles of 109.5°.
To explain this discrepancy, Linus Pauling introduced the concept of hybridization. In this model, the carbon atom’s 2s orbital mixes with its three 2p orbitals to form four equivalent sp³ hybrid orbitals. These sp³ orbitals are oriented tetrahedrally around the carbon atom, allowing for the formation of four identical sigma (σ) bonds with hydrogen atoms.
Hybridization addresses several key issues:
- Equivalent Bonds: It explains why molecules like methane have equivalent bonds, even though the atomic orbitals involved (2s and 2p) have different energies and shapes.
- Observed Geometry: It predicts and justifies the observed molecular geometries, such as the tetrahedral shape of methane or the trigonal planar shape of boron trifluoride (BF3).
- Stronger Bonds: Hybrid orbitals are more directional than atomic orbitals, leading to greater overlap with other atoms and the formation of stronger, more stable bonds.
By hybridizing atomic orbitals, atoms can achieve more stable bonding arrangements that minimize electron repulsion and maximize bond strength. This concept is fundamental to understanding the structure and properties of molecules. COMPARE.EDU.VN provides detailed comparisons and explanations to clarify these complex concepts.
2. Types of Hybrid Orbitals
Hybrid orbitals are categorized based on the number and types of atomic orbitals that combine during hybridization. The most common types are sp, sp², sp³, sp³d, and sp³d², each resulting in distinct molecular geometries.
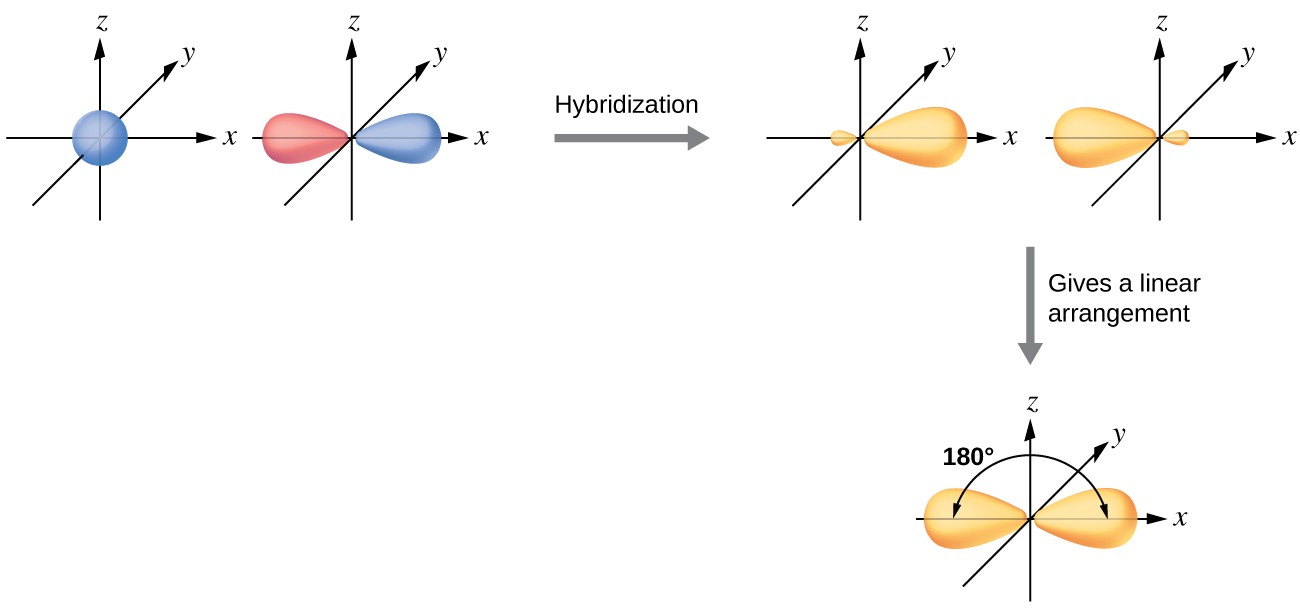
2.1 sp Hybridization
sp hybridization involves the mixing of one s orbital and one p orbital to form two sp hybrid orbitals. These two orbitals are oriented linearly, 180° apart.
- Formation: One s orbital + One p orbital → Two sp hybrid orbitals
- Geometry: Linear
- Bond Angle: 180°
- Characteristics: Each sp hybrid orbital has 50% s character and 50% p character. This high s character results in sp hybrid orbitals being lower in energy and holding electrons closer to the nucleus compared to sp² and sp³ hybrid orbitals.
- Examples: Beryllium chloride (BeCl2), carbon dioxide (CO2), ethyne (C2H2)
2.2 sp² Hybridization
sp² hybridization involves the mixing of one s orbital and two p orbitals to form three sp² hybrid orbitals. These three orbitals are arranged in a trigonal planar geometry, with each orbital 120° apart.
- Formation: One s orbital + Two p orbitals → Three sp² hybrid orbitals
- Geometry: Trigonal Planar
- Bond Angle: 120°
- Characteristics: Each sp² hybrid orbital has 33.3% s character and 66.7% p character. The remaining unhybridized p orbital can form π bonds.
- Examples: Boron trifluoride (BF3), ethene (C2H4), formaldehyde (CH2O)
2.3 sp³ Hybridization
sp³ hybridization involves the mixing of one s orbital and three p orbitals to form four sp³ hybrid orbitals. These four orbitals are oriented tetrahedrally, with each orbital 109.5° apart.
- Formation: One s orbital + Three p orbitals → Four sp³ hybrid orbitals
- Geometry: Tetrahedral
- Bond Angle: 109.5°
- Characteristics: Each sp³ hybrid orbital has 25% s character and 75% p character. This lower s character results in sp³ hybrid orbitals being higher in energy compared to sp and sp² hybrid orbitals.
- Examples: Methane (CH4), ammonia (NH3), water (H2O)
2.4 sp³d Hybridization
sp³d hybridization involves the mixing of one s orbital, three p orbitals, and one d orbital to form five sp³d hybrid orbitals. These five orbitals are arranged in a trigonal bipyramidal geometry.
- Formation: One s orbital + Three p orbitals + One d orbital → Five sp³d hybrid orbitals
- Geometry: Trigonal Bipyramidal
- Bond Angles: 90°, 120°, 180°
- Characteristics: The axial and equatorial positions in the trigonal bipyramidal structure are not equivalent, leading to variations in bond lengths and strengths.
- Examples: Phosphorus pentachloride (PCl5), sulfur tetrafluoride (SF4), chlorine trifluoride (ClF3)
2.5 sp³d² Hybridization
sp³d² hybridization involves the mixing of one s orbital, three p orbitals, and two d orbitals to form six sp³d² hybrid orbitals. These six orbitals are arranged in an octahedral geometry.
- Formation: One s orbital + Three p orbitals + Two d orbitals → Six sp³d² hybrid orbitals
- Geometry: Octahedral
- Bond Angle: 90°
- Characteristics: All six positions in the octahedral structure are equivalent, resulting in uniform bond lengths and strengths.
- Examples: Sulfur hexafluoride (SF6), iodine pentafluoride (IF5), xenon tetrafluoride (XeF4)
Understanding the types of hybrid orbitals and their characteristics is essential for predicting the shapes and properties of molecules. COMPARE.EDU.VN offers extensive comparisons and resources to aid in this understanding.
3. Energy Levels of Hybrid Orbitals Compared to Atomic Orbitals
The energy levels of hybrid orbitals are intermediate between the energy levels of the atomic orbitals from which they are formed. The extent of mixing and the proportion of s and p character in the hybrid orbitals determine their energy levels and bonding properties.
3.1 Energy Level Diagrams
To understand the energy levels, consider the following energy level diagrams that compare atomic orbitals to hybrid orbitals:
-
sp Hybridization:
- Atomic Orbitals: One s orbital (lower energy) and one p orbital (higher energy)
- Hybrid Orbitals: Two sp hybrid orbitals with energy levels intermediate between the s and p orbitals.
-
sp² Hybridization:
- Atomic Orbitals: One s orbital (lower energy) and two p orbitals (higher energy)
- Hybrid Orbitals: Three sp² hybrid orbitals with energy levels intermediate between the s and p orbitals. One unhybridized p orbital remains at its original energy level.
-
sp³ Hybridization:
- Atomic Orbitals: One s orbital (lower energy) and three p orbitals (higher energy)
- Hybrid Orbitals: Four sp³ hybrid orbitals with energy levels intermediate between the s and p orbitals.
-
sp³d Hybridization:
- Atomic Orbitals: One s orbital, three p orbitals, and one d orbital
- Hybrid Orbitals: Five sp³d hybrid orbitals with energy levels intermediate between the s, p, and d orbitals.
-
sp³d² Hybridization:
- Atomic Orbitals: One s orbital, three p orbitals, and two d orbitals
- Hybrid Orbitals: Six sp³d² hybrid orbitals with energy levels intermediate between the s, p, and d orbitals.
-
Energy Level Characteristics:
- The energy of hybrid orbitals increases with increasing p character and decreases with increasing s character.
- sp hybrid orbitals have the lowest energy due to their high s character, followed by sp², sp³, sp³d, and sp³d² hybrid orbitals.
3.2 Impact of s-Character
The s character of a hybrid orbital significantly influences its energy level and bonding properties. Higher s character means:
-
Lower Energy: Electrons in orbitals with more s character are held closer to the nucleus, resulting in lower energy.
-
Shorter Bond Length: Bonds formed by hybrid orbitals with higher s character are shorter and stronger due to the electron density being closer to the nucleus.
-
Greater Electronegativity: Atoms with hybrid orbitals of higher s character exhibit greater electronegativity.
-
Examples:
- In ethyne (C2H2), the carbon atoms are sp hybridized with 50% s character, resulting in strong, short bonds.
- In ethene (C2H4), the carbon atoms are sp² hybridized with 33.3% s character, leading to intermediate bond strength and length.
- In ethane (C2H6), the carbon atoms are sp³ hybridized with 25% s character, resulting in weaker, longer bonds.
4. Factors Affecting Hybrid Orbital Energy
Several factors can influence the energy levels of hybrid orbitals, including the electronegativity of surrounding atoms, the presence of lone pairs, and the overall molecular geometry.
4.1 Electronegativity of Surrounding Atoms
The electronegativity of atoms bonded to the central atom can affect the electron distribution and energy levels of hybrid orbitals. More electronegative atoms pull electron density away from the central atom, which can stabilize the hybrid orbitals and lower their energy levels.
- Example: In molecules like carbonyl halides (e.g., CH3COCl), the electronegative chlorine atom withdraws electron density from the carbon atom, affecting the energy levels of the sp² hybrid orbitals and the carbonyl bond.
4.2 Lone Pair Effects
Lone pairs of electrons exert a greater repulsive force than bonding pairs, which can distort the geometry of molecules and affect the energy levels of hybrid orbitals. The presence of lone pairs tends to increase the energy of hybrid orbitals.
- Example: In water (H2O), the oxygen atom is sp³ hybridized, but the two lone pairs cause a deviation from the ideal tetrahedral angle (109.5°) to a smaller angle (104.5°). The lone pairs increase the energy of the sp³ hybrid orbitals.
4.3 Molecular Geometry
The overall molecular geometry dictated by VSEPR theory influences the arrangement and energy levels of hybrid orbitals. Different geometries require different types of hybridization, each with its unique energy characteristics.
- Example: Linear molecules like CO2 require sp hybridization, which results in hybrid orbitals with lower energy due to high s character. Tetrahedral molecules like CH4 require sp³ hybridization, leading to hybrid orbitals with higher energy.
5. Applications and Examples
Understanding the energy levels of hybrid orbitals is crucial in various chemical applications, including predicting molecular properties, reaction mechanisms, and spectroscopic behavior.
5.1 Predicting Molecular Properties
The energy levels of hybrid orbitals can be used to predict various molecular properties, such as bond strength, bond length, and molecular stability.
- Bond Strength and Length: Hybrid orbitals with higher s character form stronger, shorter bonds due to the increased electron density near the nucleus.
- Molecular Stability: Molecules with lower energy hybrid orbitals are generally more stable due to the stronger bonds and reduced electron repulsion.
5.2 Reaction Mechanisms
Hybridization and the energy levels of hybrid orbitals play a vital role in understanding reaction mechanisms. Changes in hybridization during a reaction can affect the energy barriers and transition states, influencing the reaction rate and selectivity.
- SN1 and SN2 Reactions: The hybridization of carbon atoms in substrates and intermediates affects the stability of carbocations and transition states in SN1 and SN2 reactions.
- Addition Reactions: In addition reactions, the hybridization of carbon atoms in alkenes changes from sp² to sp³, affecting the energy levels and stability of the products.
5.3 Spectroscopic Behavior
The energy levels of hybrid orbitals influence the spectroscopic behavior of molecules, affecting the absorption and emission of electromagnetic radiation.
- UV-Vis Spectroscopy: The electronic transitions between hybrid orbitals and π orbitals determine the absorption spectra of organic molecules.
- NMR Spectroscopy: The chemical shifts of atoms in NMR spectra are influenced by the electron density around the nucleus, which is affected by the hybridization of the atom.
6. Advanced Concepts in Hybridization
Beyond the basic types of hybridization, several advanced concepts provide a deeper understanding of molecular bonding and electronic structure.
6.1 Isovalent Hybridization
Isovalent hybridization involves varying the s and p character of hybrid orbitals to fine-tune bond angles and minimize electron repulsion. This concept is particularly relevant in molecules with lone pairs or electronegative substituents.
- Example: In ammonia (NH3), the nitrogen atom is approximately sp³ hybridized, but the bond angle is slightly less than the ideal tetrahedral angle (109.5°). This is because the lone pair on nitrogen occupies a hybrid orbital with slightly more s character, resulting in greater repulsion and a smaller bond angle.
6.2 Bent’s Rule
Bent’s rule states that more electronegative substituents prefer to bond to hybrid orbitals with more p character, while more electropositive substituents prefer hybrid orbitals with more s character. This rule helps explain the distribution of s and p character in molecules with multiple substituents.
- Example: In fluoromethane (CH3F), the fluorine atom, being highly electronegative, prefers to bond to a hybrid orbital on carbon with more p character. This results in the other C-H bonds having slightly more s character and shorter bond lengths.
6.3 Resonance and Hybridization
Resonance structures can influence the hybridization of atoms in molecules. When a molecule has multiple resonance forms, the actual hybridization is an average of the hybridizations in the contributing resonance structures.
- Example: In the carbonate ion (CO3²⁻), the carbon atom is sp² hybridized. However, due to resonance, all three C-O bonds are equivalent, and each oxygen atom has a partial double bond character.
7. Conclusion
Understanding how the energy levels of hybrid orbitals compare to those of atomic orbitals is essential for comprehending molecular bonding, geometry, and properties. Hybrid orbitals are intermediate in energy between the atomic orbitals from which they are formed, with the s character playing a significant role in determining their energy levels. Hybridization allows atoms to form stronger, more stable bonds, resulting in predictable molecular structures and behaviors.
By exploring these concepts further on COMPARE.EDU.VN, students, consumers and professionals can gain a deeper appreciation for the intricacies of chemical bonding and molecular structure. Whether you are comparing different molecules or seeking to understand chemical reactions, the principles of hybridization provide a valuable framework for analysis and prediction. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or via Whatsapp at +1 (626) 555-9090.
COMPARE.EDU.VN provides detailed comparisons, resources, and insights to help you make informed decisions.
8. Frequently Asked Questions (FAQ)
1. What is the difference between atomic orbitals and hybrid orbitals?
Atomic orbitals are the original orbitals in an isolated atom, while hybrid orbitals are formed by mixing atomic orbitals to create new orbitals suitable for bonding. Hybrid orbitals have different shapes and energies compared to atomic orbitals.
2. Why do atoms hybridize their orbitals?
Atoms hybridize their orbitals to achieve more stable bonding arrangements that minimize electron repulsion and maximize bond strength. Hybridization explains observed molecular geometries and equivalent bond properties.
3. How does s character affect the energy of a hybrid orbital?
Higher s character in a hybrid orbital results in lower energy because electrons in orbitals with more s character are held closer to the nucleus.
4. What is the geometry associated with sp hybridization?
sp hybridization results in a linear geometry with a bond angle of 180°.
5. How do lone pairs affect the energy levels of hybrid orbitals?
Lone pairs exert greater repulsive forces than bonding pairs, which can distort molecular geometry and increase the energy of hybrid orbitals.
6. What is Bent’s rule, and how does it relate to hybridization?
Bent’s rule states that more electronegative substituents prefer to bond to hybrid orbitals with more p character, while more electropositive substituents prefer hybrid orbitals with more s character. This rule helps explain the distribution of s and p character in molecules with multiple substituents.
7. Can the hybridization of an atom change during a chemical reaction?
Yes, the hybridization of an atom can change during a chemical reaction as bonds are broken and formed, leading to changes in molecular geometry and energy levels.
8. What are some examples of molecules with sp³d² hybridization?
Examples of molecules with sp³d² hybridization include sulfur hexafluoride (SF6), iodine pentafluoride (IF5), and xenon tetrafluoride (XeF4).
9. How does resonance affect hybridization?
Resonance can influence the hybridization of atoms in molecules. When a molecule has multiple resonance forms, the actual hybridization is an average of the hybridizations in the contributing resonance structures.
10. Where can I find more information about hybrid orbitals and molecular bonding?
Visit COMPARE.EDU.VN for detailed comparisons, resources, and insights to help you understand hybrid orbitals, molecular bonding, and chemical properties.
Understanding the nuances of hybrid orbital energy levels is essential for mastering molecular chemistry. For more comprehensive comparisons and resources, visit COMPARE.EDU.VN today and make informed decisions.
Call to Action:
Ready to dive deeper into molecular comparisons? Visit compare.edu.vn now to explore detailed analyses and make informed decisions. Your journey to clarity starts here! Our address is 333 Comparison Plaza, Choice City, CA 90210, United States, and we are also available via Whatsapp at +1 (626) 555-9090.