Lithium is a fascinating element, pivotal in modern battery technology. Understanding the distinction between a lithium cation and a lithium atom is crucial, especially when exploring advancements in lithium-ion and lithium-metal batteries. This article delves into a comparison of these two forms of lithium, drawing insights from cutting-edge research on solid polymer electrolytes and ionic liquids, to highlight their unique roles and properties.
What is a Lithium Atom?
A lithium atom (Li) is the most basic form of lithium, characterized by a neutral charge. Looking at its atomic structure, a lithium atom has:
- Protons: 3 (in the nucleus, defining it as lithium)
- Neutrons: Typically 4 (in the nucleus, isotopes can vary)
- Electrons: 3 (orbiting the nucleus)
These three electrons are arranged in electron shells. Lithium has an electronic configuration of 1s²2s¹. This means it has two electrons in its inner shell (1s) and one electron in its outer shell (2s). This single electron in the outermost shell, known as the valence electron, is what dictates lithium’s chemical behavior.
What is a Lithium Cation?
A lithium cation (Li⁺), on the other hand, is a positively charged ion. It forms when a lithium atom loses its valence electron. As a result, a lithium cation has:
- Protons: 3 (still defining it as lithium)
- Neutrons: Typically 4
- Electrons: 2
The electronic configuration of a lithium cation is 1s². By losing its outer electron, it achieves a stable electron configuration, similar to helium, the nearest noble gas. This loss of an electron results in a net positive charge of +1 because there is now one more proton than electrons.
Key Differences Between Lithium Cation and Lithium Atom
The fundamental difference in electron count and charge leads to significant distinctions in their properties and behavior:
1. Charge and Electronic Configuration
- Lithium Atom (Li): Neutral charge, electronic configuration 1s²2s¹, reactive due to the single valence electron.
- Lithium Cation (Li⁺): Positive +1 charge, electronic configuration 1s², stable electron configuration, significantly less reactive than the atom.
2. Reactivity
Lithium atoms are highly reactive. They readily donate their valence electron to achieve stability, making them strong reducing agents. Lithium metal reacts vigorously with water, air, and many other substances.
Lithium cations, having already achieved a stable electron configuration, are much less reactive. They are stable in many chemical environments and exist as ions in salts and solutions.
3. Size and Ionic Radius
- Lithium Atom: Has a larger atomic radius because it includes the outer electron shell.
- Lithium Cation: Smaller ionic radius as it has lost its outer electron shell, and the remaining electrons are pulled closer to the nucleus by the positive charge.
This size difference is important in material science, especially in battery design, where ion mobility through electrolyte materials is crucial.
4. Occurrence and Applications
- Lithium Atom: Does not exist freely in nature due to its high reactivity. It is produced through chemical reactions and is used in specialized applications, including certain chemical syntheses and as the starting material for lithium compounds.
- Lithium Cation: Exists widely in nature as part of salts and minerals. It is the form of lithium crucial for biological systems and, most importantly, for lithium-ion batteries.
Lithium Cations in Battery Technology: Insights from Advanced Electrolytes
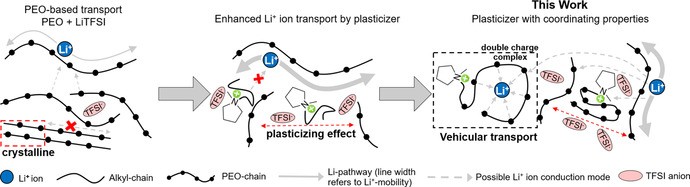
The original article ([reference to original article if needed]) focuses on enhancing lithium-ion transport in solid polymer electrolytes (SPEs) for lithium batteries. This research highlights the importance of lithium cations in electrochemical energy storage. In batteries, lithium ions are the charge carriers that move between the anode and cathode during charging and discharging.
The study introduces a novel ionic liquid, Pyr1,(2O)7TFSI, designed to improve the movement of lithium cations within the electrolyte. Traditional electrolytes using alkyl-based ionic liquids often suffer from low lithium transference numbers. This is because the added ions in the ionic liquid, which are not lithium, dilute the concentration of charge carriers and don’t effectively assist lithium-ion transport.
Alt Text: Illustration comparing lithium-ion conduction modes in PEO electrolytes with alkyl-based ILs versus coordinating Pyr1,(2O)7TFSI IL.
The innovation lies in using an ionic liquid cation with an oligo(ethylene oxide) side chain. This modification enables the cation itself to better solvate lithium ions. Solvation is the process where ions are surrounded and stabilized by molecules – in this case, the oxygen atoms in the oligo(ethylene oxide) chain interact favorably with the positive lithium cation.
This designed ionic liquid facilitates new conduction modes for lithium cations. Instead of lithium ions solely relying on the polymer chains for movement, these solvating IL cations can act as “shuttles,” carrying lithium ions and enhancing overall lithium-ion conductivity.
The research demonstrates that by using Pyr1,(2O)7TFSI, the lithium transference number is significantly increased. This means a larger fraction of the electric current is carried by lithium ions, leading to more efficient battery operation.
Alt Text: PFG-NMR measurements of self-diffusion coefficients for ionic species in Pyr1,4TFSI and Pyr1,(2O)7TFSI based polymer electrolytes, showing enhanced lithium-ion mobility with Pyr1,(2O)7TFSI.
Molecular dynamics simulations further support this, showing a correlated motion between lithium cations and these specialized IL cations, confirming the “vehicular” transport mechanism. This is a significant departure from simply increasing the mobility of polymer chains, as seen with traditional plasticizers.
Conclusion: Why the Lithium Cation’s Properties Matter
In summary, the lithium atom and lithium cation are distinctly different entities. While the lithium atom is a reactive, neutral species, the lithium cation is a stable, positively charged ion crucial for electrochemical applications.
The research on Pyr1,(2O)7TFSI highlights the importance of understanding and manipulating the properties of lithium cations to advance battery technology. By designing electrolytes that specifically enhance lithium cation transport, we can achieve higher performing, safer, and longer-lasting lithium batteries. The focus on cation solvation and conduction mechanisms represents a significant step forward in overcoming limitations of traditional solid polymer electrolytes and paving the way for next-generation lithium-metal batteries.
References (Use references from original article, properly formatted if needed, or keep as in original if markdown handles them well)
References
[1] #anie202016716-bib-0001
[2] #anie202016716-bib-0004
[3] #anie202016716-bib-0009, #anie202016716-bib-0010
[4] #anie202016716-bib-0010, #anie202016716-bib-0011
[5] #anie202016716-bib-0011
[6] #anie202016716-bib-0012
[7] #anie202016716-bib-0016
[8] #anie202016716-bib-0020
[9] #anie202016716-bib-0023
[10] #anie202016716-bib-0026
[11] #anie202016716-bib-0029
[12] #anie202016716-bib-0033
[13] #anie202016716-bib-0037, #anie202016716-bib-0038, #anie202016716-bib-0039
[14] #anie202016716-bib-0038, #anie202016716-bib-0039
[15] #anie202016716-bib-0039
[16] #anie202016716-bib-0040
[17] #anie202016716-bib-0041, #anie202016716-bib-0044, #anie202016716-bib-0045
[18] #anie202016716-bib-0044
[19] #anie202016716-bib-0045
[20] #anie202016716-bib-0046
[21] #anie202016716-bib-0047
[22] #anie202016716-bib-0048
[23] #anie202016716-bib-0053
[24] #anie202016716-bib-0056
[25] #anie202016716-bib-0057
[26] #anie202016716-bib-0061
[27] #anie202016716-bib-0065
[28] #anie202016716-bib-0068, #anie202016716-bib-0069, #anie202016716-bib-0070, #anie202016716-bib-0071
[29] #anie202016716-bib-0069, #anie202016716-bib-0070, #anie202016716-bib-0071
[30] #anie202016716-bib-0070, #anie202016716-bib-0071
[31] #anie202016716-bib-0071
[32] #anie202016716-bib-0072
[33] #anie202016716-bib-0075
[34] #anie202016716-bib-0079
[35] #anie202016716-bib-0083