Cellulose and amylose are both polysaccharides composed of glucose units, but their structural differences lead to distinct properties and functions. A key difference lies in the type of glycosidic linkage connecting the glucose monomers, which significantly impacts their three-dimensional structures and consequently, their dimensions. This article explores the comparative dimensions of cellulose and amylose, highlighting how these differences arise from their distinct molecular architectures.
Cellulose: A Linear and Fibrous Polymer
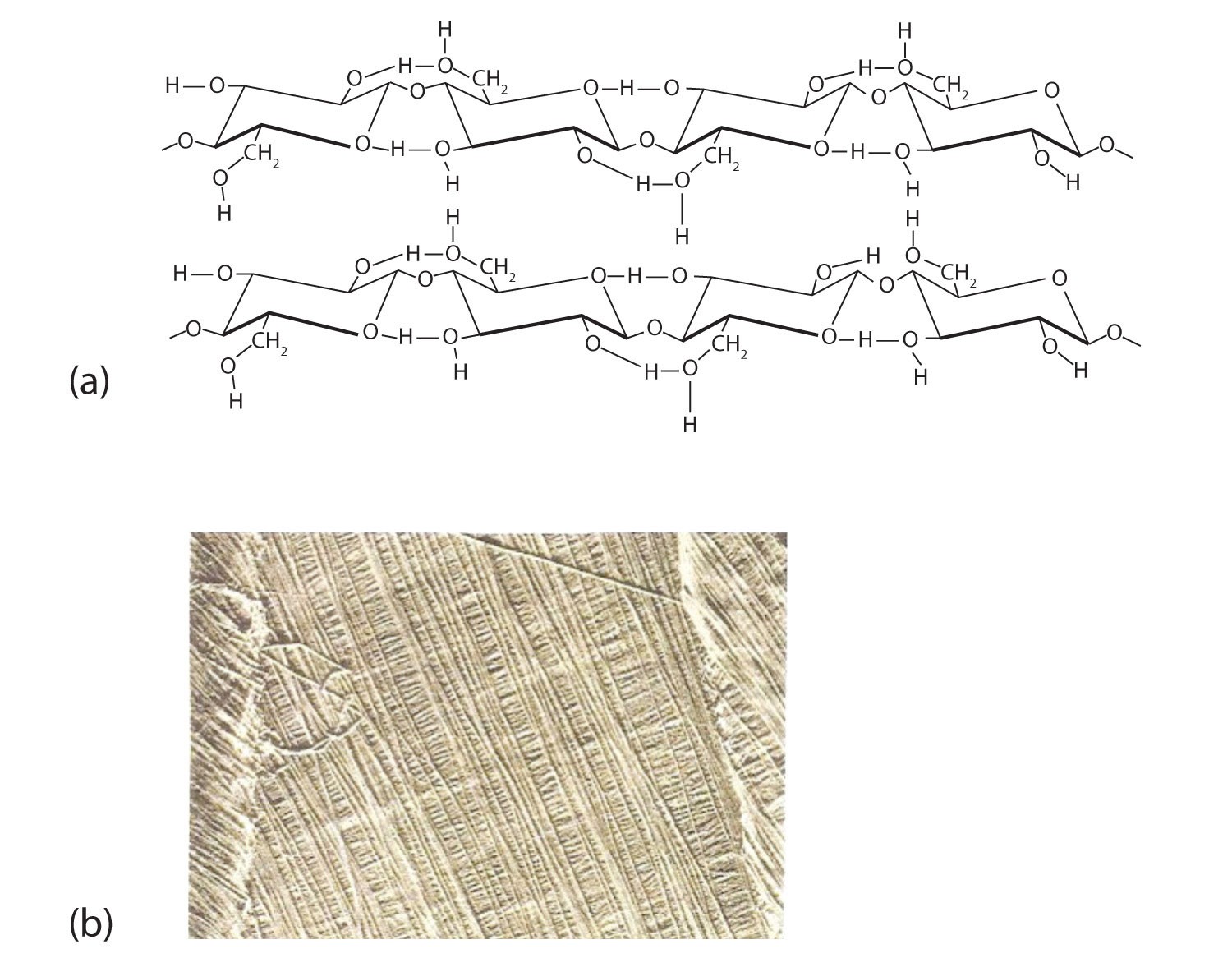
Cellulose, the most abundant organic polymer on Earth, forms the structural foundation of plant cell walls. It’s a linear polymer consisting of glucose units linked by β-1,4-glycosidic bonds. This specific linkage results in a straight, extended chain conformation.
 16.11.jpg
16.11.jpg
Figure: Cellulose structure showcasing extensive hydrogen bonding and the parallel arrangement of cellulose fibers in an algal cell wall.
The linear structure of cellulose allows for extensive hydrogen bonding between adjacent chains. These strong intermolecular forces cause the chains to pack tightly together, forming rigid, insoluble fibers with high tensile strength. This fibrous nature and insolubility are crucial for cellulose’s role in providing structural support to plants. Materials like cotton and wood derive their strength from the tightly packed cellulose fibers.
Amylose: A Helical Polymer
Amylose, a component of starch, also consists of glucose units, but they are connected by α-1,4-glycosidic bonds. This type of linkage results in a helical conformation, contrasting with the linear structure of cellulose. The helical structure of amylose is less extended and more compact compared to the straight chains of cellulose. This difference in shape influences its properties, making amylose soluble in water and allowing it to form complexes with iodine, resulting in a characteristic blue-black color. This reaction is often used to test for the presence of starch.
Dimensional Comparison: Linear vs. Helical
The β-1,4-glycosidic linkages in cellulose result in a linear, extended chain, maximizing the polymer’s length. In contrast, the α-1,4-glycosidic linkages in amylose create a helical structure, compacting the polymer and reducing its overall length compared to a fully extended chain of the same number of glucose units. While both polymers are built from the same monomer, their dimensions differ significantly due to the contrasting conformations dictated by the type of glycosidic linkage. Cellulose forms long, fibrous structures ideal for providing structural support, whereas the compact, helical structure of amylose facilitates its storage and utilization as an energy source in plants.
Conclusion
The dimensions of cellulose and amylose differ significantly due to the distinct glycosidic linkages between their glucose monomers. Cellulose’s β-1,4-linkages create a linear, extended structure leading to long, fibrous chains that contribute to its strength and insolubility. Amylose, with its α-1,4-linkages, adopts a helical conformation, resulting in a more compact structure suitable for energy storage. This fundamental difference in their three-dimensional structures underscores their distinct roles in biological systems.