The pH scale measures how acidic or basic a solution is. Ranging from 0 to 14, it provides a crucial framework for understanding chemical properties. But how do the numbers on this scale relate to each other? This article delves into the logarithmic nature of the pH scale and explains how to interpret its values.
Source: Wikipedia
Understanding the Logarithmic Nature of the pH Scale
The pH scale isn’t linear; it’s logarithmic. This means each whole number change on the scale represents a tenfold difference in acidity or basicity. For instance, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a hundred times more acidic than a solution with a pH of 6. Conversely, a pH of 9 is ten times more basic than a pH of 8.
Decoding pH Values
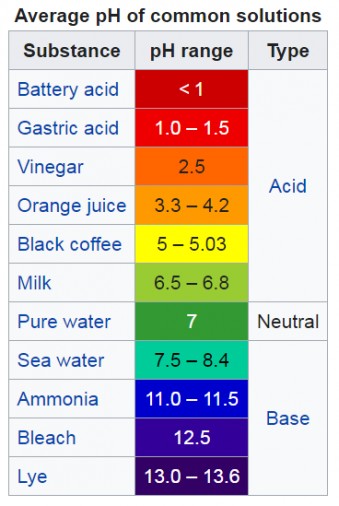
- 0-6: Indicates increasing acidity. Lower numbers represent stronger acids.
- 7: Represents neutral, like pure water.
- 8-14: Indicates increasing alkalinity (basicity). Higher numbers represent stronger bases.
What Does pH Measure?
pH measures the concentration of hydrogen ions (H+) in a solution. Acids release H+ ions when dissolved in water, while bases accept them. A higher H+ concentration corresponds to a lower pH (more acidic), and a lower H+ concentration corresponds to a higher pH (more basic).
Calculating pH
pH is calculated using the following formula:
pH = -log[H3O+]
This formula calculates the negative logarithm of the hydronium ion (H3O+) concentration, which is essentially the same as the H+ concentration in aqueous solutions. Calculating pH for strong acids is straightforward because they fully dissociate in water. For weak acids, the calculation is more complex because they don’t fully dissociate.
pH Indicators and Charts
pH indicators offer a visual way to estimate pH. These substances change color depending on the pH of the solution. pH indicator charts display the color changes associated with different pH ranges, allowing for quick, albeit less precise, pH estimations.
Source: Science Notes
Real-World Applications of pH
Understanding pH is crucial in various fields:
- Environmental Monitoring: Measuring pH helps assess water and soil quality, impacting ecosystem health and pollution control.
- Biological Systems: Maintaining a specific pH is critical for proper bodily function, impacting blood chemistry, enzyme activity, and overall health.
- Industrial Processes: pH control is essential in food preservation, water treatment, and chemical manufacturing, ensuring product quality and safety.
Conclusion
The pH scale provides a crucial framework for comparing the acidity and basicity of solutions. Its logarithmic nature allows for a wide range of values to be expressed concisely. Understanding how numbers on the pH scale compare is fundamental to various scientific and practical applications, from environmental monitoring to industrial processes and human health.