Exergonic and endergonic reactions are fundamental concepts in thermodynamics, describing energy changes in chemical reactions, and this article at COMPARE.EDU.VN provides a detailed comparison. Exergonic reactions release energy, while endergonic reactions require energy input to proceed. By understanding the nuances of these reactions, including activation energy, spontaneity, and the role of catalysts, you can gain a deeper insight into the energetic processes driving the world around us. Key differentiators include Gibbs free energy, spontaneity, and energy requirements.
1. Understanding Exergonic Reactions: Energy Release
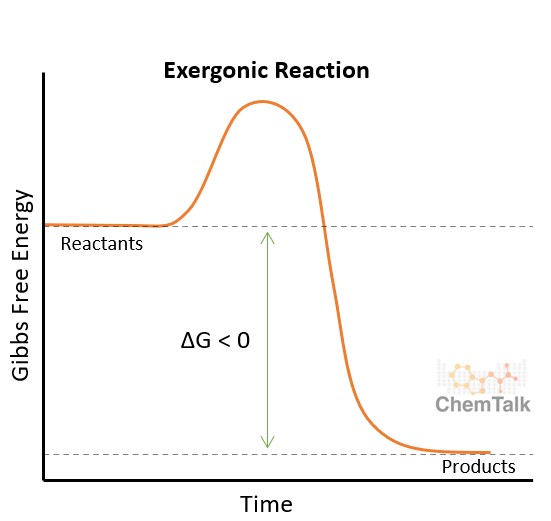
Exergonic reactions are characterized by the release of free energy, meaning the products have lower energy than the reactants. This energy release makes the reaction favorable or spontaneous, implying it can occur without external energy input. COMPARE.EDU.VN highlights that, despite this spontaneity, the reaction rate can vary significantly.
1.1. Gibbs Free Energy in Exergonic Reactions
Gibbs free energy (ΔG) is the measure of the amount of energy available in a chemical or physical system to do useful work at a constant temperature and pressure. In exergonic reactions, ΔG is negative, signifying that energy is released into the surroundings.
1.2. Spontaneity of Exergonic Reactions
The term “spontaneous” in chemistry refers to a reaction’s ability to occur without needing external energy. Exergonic reactions are spontaneous due to the release of energy, which drives the reaction forward. However, this doesn’t dictate the speed of the reaction. For example, the rusting of iron is exergonic but happens slowly.
1.3. Examples of Exergonic Reactions
Exergonic reactions occur everywhere. One notable example is glycolysis, where glucose breaks down into pyruvate, releasing energy for the body to use. Another instance is the reaction between sodium and chlorine to produce table salt (sodium chloride), which releases significant energy.
2. Delving into Endergonic Reactions: Energy Input
Endergonic reactions contrast with exergonic reactions, requiring energy input to proceed. The products in these reactions have higher energy than the reactants, making them unfavorable or non-spontaneous unless energy is supplied. COMPARE.EDU.VN notes that supplying energy can be done through heat or coupling with exergonic reactions.
2.1. Gibbs Free Energy in Endergonic Reactions
In endergonic reactions, the Gibbs free energy (ΔG) is positive, indicating that energy must be absorbed from the surroundings for the reaction to occur.
2.2. Non-Spontaneity of Endergonic Reactions
Endergonic reactions are non-spontaneous because they need external energy to proceed. This energy input overcomes the energy barrier, allowing the reaction to proceed from reactants to products.
2.3. Examples of Endergonic Reactions
Endergonic reactions are common in nature. Photosynthesis, where plants convert carbon dioxide and water into sugar and oxygen using sunlight, is a prime example. Another basic example is melting ice, which requires heat energy from the surroundings.
3. Key Differences Between Exergonic and Endergonic Reactions
Understanding the contrast between exergonic and endergonic reactions is vital in grasping chemical thermodynamics. The fundamental differences lie in energy flow, spontaneity, and Gibbs free energy change. COMPARE.EDU.VN provides a comparison to highlight these variations.
3.1. Energy Flow
- Exergonic Reactions: Release energy into the surroundings.
- Endergonic Reactions: Absorb energy from the surroundings.
3.2. Spontaneity
- Exergonic Reactions: Spontaneous, occurring without external energy input.
- Endergonic Reactions: Non-spontaneous, needing energy input to proceed.
3.3. Gibbs Free Energy (ΔG)
- Exergonic Reactions: Have a negative ΔG, indicating energy release.
- Endergonic Reactions: Have a positive ΔG, indicating energy absorption.
4. Spontaneity in Detail: Favoring Products or Reactants
Spontaneity in chemical reactions refers to whether the reaction favors the formation of products or reactants. Exergonic reactions favor products, while endergonic reactions favor reactants unless energy is supplied. COMPARE.EDU.VN points out that spontaneity should not be confused with reaction speed.
4.1. Exergonic Reactions: Favoring Products
Exergonic reactions are spontaneous because the products have lower energy than the reactants. This energy difference drives the reaction towards product formation.
4.2. Endergonic Reactions: Favoring Reactants
Endergonic reactions favor reactants over products unless energy is added. The higher energy state of the products means the reaction will not proceed without an external energy source.
4.3. Examples Illustrating Spontaneity
The oxidation of iron (rusting) is a classic example of an exergonic reaction that is spontaneous but slow. Photosynthesis, conversely, is an endergonic reaction that requires continuous energy input from sunlight to occur.
5. Exothermic vs. Endothermic Reactions: The Role of Heat
Exothermic and endothermic reactions are frequently confused with exergonic and endergonic reactions. The key distinction is that exothermic and endothermic reactions specifically describe heat energy changes. COMPARE.EDU.VN clarifies that while related, these terms are not interchangeable.
5.1. Exothermic Reactions: Heat Release
Exothermic reactions release heat, increasing the temperature of the surroundings. These reactions are a subset of exergonic reactions where the released energy is in the form of heat.
5.2. Endothermic Reactions: Heat Absorption
Endothermic reactions absorb heat, decreasing the temperature of the surroundings. These reactions are a subset of endergonic reactions where the energy absorbed is in the form of heat.
5.3. Relationship to Exergonic and Endergonic Reactions
- All exothermic reactions are exergonic if heat is the only form of energy released.
- All endothermic reactions are endergonic if heat is the only form of energy absorbed.
6. Activation Energy: Starting the Reaction
Activation energy is the minimum energy required to start a chemical reaction. It’s the energy barrier that must be overcome for reactants to transform into products. COMPARE.EDU.VN explains that both exergonic and endergonic reactions require activation energy.
6.1. Definition of Activation Energy
Activation energy is the energy needed to reach the transition state, the highest energy point in the reaction pathway.
6.2. Role in Exergonic Reactions
Even though exergonic reactions are spontaneous, they still need activation energy to start. This initial energy input is necessary to break existing bonds and begin forming new ones.
6.3. Role in Endergonic Reactions
Endergonic reactions also require activation energy, in addition to the energy needed to overcome the positive Gibbs free energy change.
7. Catalysts: Lowering Activation Energy
Catalysts are substances that speed up chemical reactions by lowering the activation energy. They do not change the Gibbs free energy of the reaction but provide an alternative reaction pathway with a lower energy barrier.
7.1. How Catalysts Work
Catalysts work by forming temporary bonds with the reactants, stabilizing the transition state, and thus reducing the activation energy needed for the reaction to proceed.
7.2. Effect on Exergonic Reactions
In exergonic reactions, catalysts help the reaction proceed faster by lowering the activation energy. This means more reactant molecules have sufficient energy to overcome the energy barrier, leading to a quicker reaction rate.
7.3. Effect on Endergonic Reactions
Similarly, catalysts help endergonic reactions proceed more efficiently by lowering the activation energy. This makes it easier to supply the necessary energy for the reaction to occur.
8. Coupling Reactions: Harnessing Energy
Coupling reactions involves linking an endergonic reaction with an exergonic reaction to drive the endergonic reaction forward. The energy released by the exergonic reaction is used to fuel the endergonic reaction.
8.1. The Principle of Coupling
The principle relies on the fact that the overall Gibbs free energy change for the coupled reaction must be negative for the process to be spontaneous.
8.2. Examples of Coupled Reactions
A common example is the coupling of ATP hydrolysis (exergonic) with various cellular processes (endergonic), such as muscle contraction and protein synthesis.
8.3. Importance in Biological Systems
Coupling reactions are crucial in biological systems, allowing cells to perform energy-demanding tasks by using the energy from ATP hydrolysis.
9. Real-World Applications of Exergonic and Endergonic Reactions
Exergonic and endergonic reactions are fundamental to various processes in industry, nature, and everyday life. Understanding these reactions allows us to innovate and improve technologies and processes.
9.1. Industrial Applications
In industry, exergonic reactions are used in the production of energy and synthesis of various chemicals. Endergonic reactions are used in the production of complex compounds that require energy input.
9.2. Natural Processes
In nature, exergonic reactions drive processes like decomposition and combustion, while endergonic reactions such as photosynthesis support life by converting light energy into chemical energy.
9.3. Everyday Life
In everyday life, exergonic reactions are seen in burning fuel for heat, while endergonic reactions are utilized in cooking and food preservation.
10. Gibbs Free Energy: A Thermodynamic Compass
Gibbs free energy (ΔG) is a crucial concept in thermodynamics, providing a measure of the potential for reversible or maximum work that can be done by a system at constant temperature and pressure. Understanding ΔG allows us to predict the spontaneity of a reaction.
10.1. Definition and Significance
Gibbs free energy combines enthalpy (heat content) and entropy (disorder) to determine the spontaneity of a reaction. A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a non-spontaneous reaction.
10.2. Calculation of ΔG
The Gibbs free energy change can be calculated using the equation: ΔG = ΔH – TΔS, where ΔH is the enthalpy change, T is the temperature in Kelvin, and ΔS is the entropy change.
10.3. Factors Affecting ΔG
Factors such as temperature, pressure, and concentration of reactants and products can affect the Gibbs free energy change and, therefore, the spontaneity of a reaction.
11. Entropy: The Measure of Disorder
Entropy, often denoted as S, is a measure of the disorder or randomness of a system. In thermodynamics, entropy plays a vital role in determining the spontaneity of reactions.
11.1. Definition and Significance
A system with higher entropy is more disordered, while a system with lower entropy is more ordered. The change in entropy (ΔS) is crucial in determining the Gibbs free energy change.
11.2. Entropy Changes in Reactions
In chemical reactions, entropy can increase or decrease. Reactions that produce more gaseous molecules or simpler molecules typically have an increase in entropy.
11.3. Relationship to Spontaneity
An increase in entropy (positive ΔS) favors spontaneity, while a decrease in entropy (negative ΔS) opposes spontaneity.
12. Enthalpy: The Heat Content
Enthalpy, denoted as H, is the heat content of a system at constant pressure. The change in enthalpy (ΔH) is another critical factor in determining the spontaneity of a reaction.
12.1. Definition and Significance
Enthalpy represents the total heat content of a system and is commonly used to describe the heat absorbed or released during a chemical reaction.
12.2. Enthalpy Changes in Reactions
Exothermic reactions have a negative ΔH, indicating heat is released, while endothermic reactions have a positive ΔH, indicating heat is absorbed.
12.3. Relationship to Spontaneity
A decrease in enthalpy (negative ΔH) favors spontaneity, while an increase in enthalpy (positive ΔH) opposes spontaneity.
13. The Role of Temperature
Temperature plays a significant role in determining the spontaneity of reactions, particularly in those where both enthalpy and entropy changes are significant.
13.1. Temperature Dependence of ΔG
The Gibbs free energy equation (ΔG = ΔH – TΔS) shows that temperature directly affects the contribution of entropy to the spontaneity of a reaction.
13.2. High Temperatures
At high temperatures, the entropy term (TΔS) becomes more significant, favoring reactions with a positive ΔS (increase in entropy).
13.3. Low Temperatures
At low temperatures, the enthalpy term (ΔH) becomes more significant, favoring reactions with a negative ΔH (decrease in enthalpy).
14. Pressure and Volume Effects
Pressure and volume can also influence the spontaneity of reactions, especially those involving gases.
14.1. Pressure Effects
Increasing the pressure can favor reactions that decrease the number of gas molecules, as this reduces the volume and lowers the energy of the system.
14.2. Volume Effects
Decreasing the volume can also favor reactions that decrease the number of gas molecules for the same reasons.
14.3. Le Chatelier’s Principle
Le Chatelier’s Principle states that if a system at equilibrium is subjected to a change in conditions, such as pressure or volume, the system will adjust to counteract the change and restore a new equilibrium.
15. Concentration and Reaction Quotient
The concentration of reactants and products can also affect the spontaneity of a reaction. The reaction quotient (Q) is a measure of the relative amount of products and reactants present in a reaction at a given time.
15.1. The Reaction Quotient (Q)
Q indicates the direction a reversible reaction must shift to reach equilibrium.
15.2. Relationship to Equilibrium Constant (K)
The Gibbs free energy change can be related to the equilibrium constant (K) using the equation: ΔG = -RTlnK, where R is the gas constant and T is the temperature in Kelvin.
15.3. Predicting Reaction Direction
By comparing Q to K, we can predict whether a reaction will proceed forward (Q < K), backward (Q > K), or is at equilibrium (Q = K).
16. Equilibrium: The Balance Point
Equilibrium is the state in which the rate of the forward reaction equals the rate of the reverse reaction, and there is no net change in the concentrations of reactants and products.
16.1. Dynamic Equilibrium
Equilibrium is dynamic, meaning that reactions are still occurring, but the rates are equal in both directions.
16.2. Equilibrium Constant (K)
The equilibrium constant (K) is a measure of the relative amounts of products and reactants at equilibrium. A large K indicates that the equilibrium favors products, while a small K indicates that the equilibrium favors reactants.
16.3. Factors Affecting Equilibrium
Factors such as temperature, pressure, and concentration can affect the equilibrium position, as described by Le Chatelier’s Principle.
17. Standard Conditions and Standard Free Energy Change
Standard conditions are a set of reference conditions used for thermodynamic calculations. The standard free energy change (ΔG°) is the change in Gibbs free energy when a reaction occurs under standard conditions.
17.1. Definition of Standard Conditions
Standard conditions are typically defined as 298 K (25 °C) and 1 atm pressure, with all reactants and products at a concentration of 1 M.
17.2. Calculating ΔG°
The standard free energy change can be calculated using the equation: ΔG° = -RTlnK, where R is the gas constant, T is the temperature in Kelvin, and K is the equilibrium constant under standard conditions.
17.3. Importance in Thermodynamics
ΔG° provides a reference point for comparing the spontaneity of different reactions and for calculating the free energy change under non-standard conditions.
18. Non-Standard Conditions: Adjusting for Reality
Most reactions do not occur under standard conditions. To calculate the Gibbs free energy change under non-standard conditions, we use the equation:
18.1. The Nernst Equation
ΔG = ΔG° + RTlnQ, where Q is the reaction quotient.
18.2. Applying the Nernst Equation
This equation allows us to adjust for the effects of concentration and pressure on the spontaneity of a reaction.
18.3. Real-World Scenarios
In real-world scenarios, such as biological systems or industrial processes, reactions rarely occur under standard conditions, making the Nernst equation essential for accurate predictions.
19. Biological Relevance: Metabolic Pathways
Exergonic and endergonic reactions are essential in biological systems, driving metabolic pathways that sustain life.
19.1. Catabolic Pathways
Catabolic pathways break down complex molecules into simpler ones, releasing energy. These pathways are exergonic and provide the energy needed for cellular processes.
19.2. Anabolic Pathways
Anabolic pathways build complex molecules from simpler ones, requiring energy input. These pathways are endergonic and use the energy released by catabolic pathways.
19.3. ATP: The Energy Currency
ATP (adenosine triphosphate) is the primary energy currency of cells. The hydrolysis of ATP is an exergonic reaction that provides the energy needed for various cellular processes, including muscle contraction, nerve impulse transmission, and protein synthesis.
20. Environmental Impact: Sustainable Chemistry
Understanding exergonic and endergonic reactions is also crucial for developing sustainable chemistry practices.
20.1. Green Chemistry
Green chemistry aims to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances.
20.2. Energy Efficiency
Designing reactions that are more energy-efficient, using less energy input or producing more energy output, can reduce the environmental impact of chemical processes.
20.3. Renewable Resources
Utilizing renewable resources as starting materials can also contribute to sustainable chemistry by reducing our reliance on fossil fuels.
21. Future Directions: Research and Innovation
Research into exergonic and endergonic reactions continues to drive innovation in various fields, including energy storage, catalysis, and materials science.
21.1. Energy Storage
Developing new materials and technologies for energy storage, such as batteries and fuel cells, relies on understanding and optimizing exergonic and endergonic reactions.
21.2. Catalysis
Research into new catalysts can lead to more efficient and selective chemical reactions, reducing waste and energy consumption.
21.3. Materials Science
Understanding the thermodynamics of materials can help in the design of new materials with specific properties, such as high strength, low weight, or superconductivity.
22. Summarizing Exergonic and Endergonic Reactions
Exergonic and endergonic reactions are fundamental concepts in thermodynamics, describing energy changes in chemical reactions. Exergonic reactions release energy, while endergonic reactions require energy input.
22.1. Key Concepts
- Exergonic reactions have a negative Gibbs free energy change (ΔG < 0) and are spontaneous.
- Endergonic reactions have a positive Gibbs free energy change (ΔG > 0) and are non-spontaneous.
- Activation energy is required to start both exergonic and endergonic reactions.
- Catalysts lower the activation energy, speeding up reactions.
- Coupling reactions involves linking an endergonic reaction with an exergonic reaction to drive the endergonic reaction forward.
22.2. Importance in Science and Technology
Understanding exergonic and endergonic reactions is crucial in various fields, including chemistry, biology, engineering, and environmental science.
22.3. Future Perspectives
Continued research into these reactions will drive innovation in energy storage, catalysis, materials science, and sustainable chemistry.
23. Common Misconceptions
It’s essential to address common misconceptions regarding exergonic and endergonic reactions to ensure a clear understanding of these concepts.
23.1. Spontaneity vs. Rate
A common misconception is that spontaneous reactions are fast. Spontaneity refers to whether a reaction can occur without external energy input, not how quickly it occurs.
23.2. Exergonic = Exothermic?
While exothermic reactions are a subset of exergonic reactions, not all exergonic reactions are exothermic. Exergonic reactions release energy in any form, while exothermic reactions specifically release heat.
23.3. Endergonic Reactions Never Happen Alone
Endergonic reactions can occur alone if sufficient energy is supplied, such as through heat or light. They do not always need to be coupled with exergonic reactions.
24. How COMPARE.EDU.VN Can Help You
COMPARE.EDU.VN offers detailed comparisons of various scientific concepts, helping students, researchers, and professionals gain a deeper understanding of complex topics like exergonic and endergonic reactions.
24.1. Comprehensive Articles
COMPARE.EDU.VN provides comprehensive articles that cover the key concepts, differences, and applications of exergonic and endergonic reactions.
24.2. Visual Aids
Visual aids, such as graphs and diagrams, help to illustrate the energy changes and processes involved in these reactions.
24.3. Expert Insights
Expert insights and explanations ensure that the information is accurate, up-to-date, and easy to understand.
25. Practical Examples and Exercises
To reinforce your understanding, consider these practical examples and exercises:
25.1. Identifying Reaction Types
Determine whether the following reactions are exergonic or endergonic:
- Combustion of methane
- Formation of glucose from carbon dioxide and water
- Hydrolysis of ATP
25.2. Calculating ΔG
Calculate the Gibbs free energy change for a reaction given the enthalpy and entropy changes at a specific temperature.
25.3. Designing Coupled Reactions
Design a coupled reaction to drive an endergonic process forward.
26. Advanced Topics: Quantum Mechanics and Reaction Rates
For those looking to delve deeper, exploring the quantum mechanical aspects of reaction rates provides a more complete understanding.
26.1. Transition State Theory
Transition state theory describes the rate of a reaction in terms of the properties of the transition state.
26.2. Quantum Tunneling
Quantum tunneling allows particles to pass through energy barriers, affecting reaction rates.
26.3. Molecular Dynamics Simulations
Molecular dynamics simulations can be used to model and predict the behavior of chemical reactions.
27. Resources for Further Learning
To continue your learning journey, explore these additional resources:
27.1. Textbooks
- “Physical Chemistry” by Peter Atkins
- “Thermodynamics and an Introduction to Thermostatistics” by Herbert B. Callen
27.2. Online Courses
- Coursera: “Chemical Thermodynamics”
- edX: “Principles of Chemical Science”
27.3. Scientific Journals
- Journal of Physical Chemistry
- Journal of Chemical Thermodynamics
28. The Future of Energy: Exergonic and Endergonic Reactions
As the world faces increasing energy demands and environmental concerns, understanding and harnessing exergonic and endergonic reactions will be crucial for developing sustainable energy solutions.
28.1. Renewable Energy Sources
Renewable energy sources, such as solar, wind, and hydro, rely on natural exergonic and endergonic reactions to generate electricity.
28.2. Energy Storage Technologies
Energy storage technologies, such as batteries and fuel cells, utilize chemical reactions to store and release energy on demand.
28.3. Carbon Capture and Utilization
Carbon capture and utilization technologies aim to capture carbon dioxide from the atmosphere and convert it into valuable products, often through endergonic reactions powered by renewable energy.
29. Innovations in Catalysis: Speeding Up Reactions
Catalysis plays a crucial role in many industrial processes, allowing reactions to occur faster and more efficiently.
29.1. Homogeneous Catalysis
Homogeneous catalysts are in the same phase as the reactants.
29.2. Heterogeneous Catalysis
Heterogeneous catalysts are in a different phase from the reactants.
29.3. Enzymatic Catalysis
Enzymes are biological catalysts that are highly specific and efficient.
30. Applying Thermodynamics to Real-World Problems
Thermodynamics is not just a theoretical science; it has practical applications in many areas of engineering and technology.
30.1. Chemical Engineering
Chemical engineers use thermodynamics to design and optimize chemical processes.
30.2. Mechanical Engineering
Mechanical engineers use thermodynamics to design engines and power plants.
30.3. Environmental Engineering
Environmental engineers use thermodynamics to design pollution control systems.
By understanding exergonic and endergonic reactions, you can gain a deeper appreciation for the fundamental processes that drive the world around us. From the smallest cellular processes to the largest industrial operations, these reactions play a vital role in shaping our lives and our planet.
Are you struggling to compare complex concepts and make informed decisions? Visit COMPARE.EDU.VN at our physical location: 333 Comparison Plaza, Choice City, CA 90210, United States, or reach out via Whatsapp at +1 (626) 555-9090. Our comprehensive comparisons and expert insights will empower you to choose the best option for your needs. Don’t hesitate—explore compare.edu.vn today!
FAQ: Exergonic and Endergonic Reactions
Q1: What is the main difference between exergonic and endergonic reactions?
Exergonic reactions release energy, while endergonic reactions require energy input to proceed.
Q2: How does Gibbs free energy relate to exergonic and endergonic reactions?
Exergonic reactions have a negative Gibbs free energy change (ΔG < 0), while endergonic reactions have a positive Gibbs free energy change (ΔG > 0).
Q3: Are exergonic reactions always spontaneous?
Yes, exergonic reactions are spontaneous because they release energy.
Q4: Do endergonic reactions always need to be coupled with exergonic reactions?
No, endergonic reactions can occur alone if sufficient energy is supplied, such as through heat or light.
Q5: What is activation energy?
Activation energy is the minimum energy required to start a chemical reaction.
Q6: How do catalysts affect exergonic and endergonic reactions?
Catalysts lower the activation energy, speeding up both exergonic and endergonic reactions.
Q7: What is the difference between exothermic and endothermic reactions?
Exothermic reactions release heat, while endothermic reactions absorb heat.
Q8: How does temperature affect the spontaneity of reactions?
Temperature can affect the spontaneity of reactions, particularly those where both enthalpy and entropy changes are significant.
Q9: What is ATP, and why is it important in biological systems?
ATP (adenosine triphosphate) is the primary energy currency of cells. The hydrolysis of ATP is an exergonic reaction that provides the energy needed for various cellular processes.
Q10: How can understanding exergonic and endergonic reactions help in developing sustainable chemistry practices?
Understanding these reactions can help in designing energy-efficient processes, utilizing renewable resources, and reducing the environmental impact of chemical processes.