Do ionic compounds have high melting points? Compare.EDU.VN answers this by exploring the crucial differences between ionic and covalent compounds. Understanding these variations allows for informed decision-making in various scientific and industrial applications, providing clarity and promoting smarter choices using the insights available on COMPARE.EDU.VN. This comparison involves properties, melting points, and compound analysis.
1. Introduction: Ionic vs. Covalent Compounds: A Comparative Overview
Ionic and covalent compounds represent two fundamental classes of chemical substances, each characterized by distinct bonding mechanisms that dictate their physical and chemical properties. Ionic compounds, formed through electrostatic interactions between oppositely charged ions, typically exhibit high melting points, robust crystalline structures, and electrical conductivity when dissolved in polar solvents. In contrast, covalent compounds, where atoms share electrons to achieve stability, generally possess lower melting points, diverse physical states at room temperature, and limited electrical conductivity. This extensive article delves into a comprehensive comparison of these two compound types, focusing specifically on the factors contributing to the higher melting points observed in ionic compounds. By examining the nature of chemical bonds, intermolecular forces, and structural arrangements, we aim to provide a detailed understanding of why ionic compounds typically require more energy to transition from a solid to a liquid state compared to their covalent counterparts. Such insights are invaluable for a wide range of applications, from materials science and chemical engineering to pharmaceutical development and environmental chemistry.
2. Understanding Chemical Bonds: The Foundation of Compound Properties
2.1. Ionic Bonds: Electrostatic Attraction and Lattice Energy
Ionic bonds arise from the transfer of electrons between atoms with significantly different electronegativities, typically between metals and nonmetals. This electron transfer results in the formation of positively charged ions (cations) and negatively charged ions (anions). The strong electrostatic attraction between these oppositely charged ions constitutes the ionic bond. A key factor influencing the properties of ionic compounds is lattice energy, defined as the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. Higher lattice energy indicates stronger ionic bonds and, consequently, higher melting points. The magnitude of lattice energy is directly proportional to the charge of the ions and inversely proportional to the distance between them. For instance, compounds with divalent ions (e.g., MgO with Mg²⁺ and O²⁻) generally have higher lattice energies and melting points compared to those with monovalent ions (e.g., NaCl with Na⁺ and Cl⁻).
2.2. Covalent Bonds: Sharing Electrons and Intermolecular Forces
Covalent bonds, on the other hand, involve the sharing of electrons between atoms, typically nonmetals, to achieve a stable electron configuration. Unlike ionic bonds, where electrostatic attraction is the dominant force, covalent compounds are held together by intermolecular forces, which are weaker attractive forces between molecules. These forces include London dispersion forces, dipole-dipole interactions, and hydrogen bonds. London dispersion forces are present in all covalent compounds and arise from temporary fluctuations in electron distribution, creating transient dipoles. Dipole-dipole interactions occur between polar molecules with permanent dipoles, while hydrogen bonds are particularly strong dipole-dipole interactions involving hydrogen atoms bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine. The strength of these intermolecular forces significantly influences the physical properties of covalent compounds, including their melting points.
3. Melting Points: A Key Differentiating Factor
3.1. High Melting Points of Ionic Compounds: The Role of Strong Electrostatic Forces
Ionic compounds are renowned for their high melting points, a direct consequence of the strong electrostatic forces holding the ions together in a crystal lattice. Overcoming these forces requires substantial energy input, translating into elevated melting temperatures. For example, sodium chloride (NaCl), a common ionic compound, has a melting point of 801°C (1474°F). This high melting point is due to the strong attraction between Na⁺ and Cl⁻ ions in the crystal lattice. Similarly, magnesium oxide (MgO), with even higher charges on its ions (Mg²⁺ and O²⁻), boasts an even higher melting point of 2852°C (5166°F). The strong electrostatic interactions in ionic compounds result in stable, rigid structures that resist thermal disruption, hence the high melting points.
3.2. Lower Melting Points of Covalent Compounds: The Influence of Weaker Intermolecular Forces
In contrast, covalent compounds generally exhibit lower melting points because the intermolecular forces holding the molecules together are weaker than the electrostatic forces in ionic compounds. For instance, methane (CH₄), a simple covalent compound, has a melting point of -182.5°C (-296.5°F). The weak London dispersion forces between methane molecules are easily overcome with minimal energy input. Water (H₂O), while possessing hydrogen bonds, has a melting point of 0°C (32°F), which is still significantly lower than that of most ionic compounds. Even larger covalent molecules with stronger intermolecular forces, such as sucrose (C₁₂H₂₂O₁₁), have melting points around 186°C (367°F), far below those of ionic compounds. The relatively weak intermolecular forces in covalent compounds allow molecules to move more freely with less energy, leading to lower melting points.
4. Factors Affecting Melting Points: A Detailed Analysis
4.1. Charge and Size of Ions: Impact on Lattice Energy
In ionic compounds, the charge and size of ions are critical determinants of lattice energy and, consequently, melting points. Higher charges on ions lead to stronger electrostatic attractions and higher lattice energies. For example, the melting point of MgO (2852°C) is much higher than that of NaCl (801°C) because Mg²⁺ and O²⁻ ions have higher charges than Na⁺ and Cl⁻ ions. Additionally, smaller ions result in shorter interionic distances and stronger attractions. Lithium fluoride (LiF) has a higher melting point than sodium iodide (NaI) due to the smaller sizes of Li⁺ and F⁻ ions compared to Na⁺ and I⁻ ions. The interplay of charge and size directly influences the strength of ionic bonds and the thermal stability of the crystal lattice.
4.2. Type and Strength of Intermolecular Forces: Influence on Covalent Compound Melting Points
For covalent compounds, the type and strength of intermolecular forces dictate the melting points. Compounds with only London dispersion forces, such as methane (CH₄) and ethane (C₂H₆), have the lowest melting points. Compounds with dipole-dipole interactions, such as acetone (CH₃COCH₃), have higher melting points than those with only London dispersion forces due to the additional electrostatic attraction between polar molecules. Hydrogen bonds significantly elevate melting points in compounds like water (H₂O) and ethanol (C₂H₅OH). However, even with hydrogen bonds, the melting points of covalent compounds are generally lower than those of ionic compounds due to the fundamental difference in bonding mechanisms. The cumulative effect of these intermolecular forces determines the energy required to overcome molecular attractions and induce melting.
5. Structural Differences: Crystalline Lattices vs. Discrete Molecules
5.1. Ionic Compounds: Ordered Crystalline Structures
Ionic compounds typically form highly ordered crystalline structures. In these lattices, each ion is surrounded by ions of opposite charge, maximizing electrostatic attractions and creating a stable, rigid arrangement. This extended network of strong ionic bonds contributes to the high melting points of ionic compounds. The energy required to disrupt this organized structure and allow ions to move freely is substantial, necessitating high temperatures. The three-dimensional arrangement of ions in a crystal lattice provides additional stability and resistance to thermal disruption.
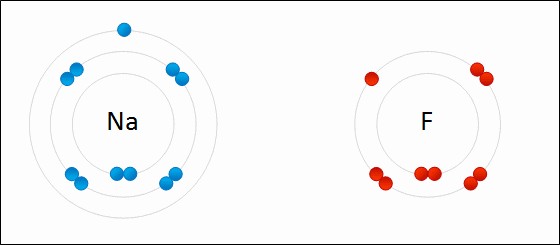 Sodium Fluoride Ionic Bond
Sodium Fluoride Ionic Bond
5.2. Covalent Compounds: Discrete Molecules with Varied Arrangements
Covalent compounds, conversely, exist as discrete molecules with relatively weak intermolecular forces between them. These molecules can be arranged in various ways, depending on their shape and polarity. Some covalent compounds form amorphous solids with disordered arrangements, while others form crystalline solids with more organized structures. However, even in crystalline covalent solids, the intermolecular forces are weaker than the ionic bonds in ionic lattices, leading to lower melting points. The ease with which molecules can move and rearrange determines the melting point, with weaker intermolecular forces facilitating melting at lower temperatures.
6. Examples and Case Studies: Illustrating the Melting Point Differences
6.1. Comparing NaCl and Sucrose: A Classic Example
A classic example illustrating the melting point difference between ionic and covalent compounds is the comparison of sodium chloride (NaCl) and sucrose (C₁₂H₂₂O₁₁). NaCl, an ionic compound, has a melting point of 801°C (1474°F), while sucrose, a covalent compound, melts at around 186°C (367°F). The high melting point of NaCl is attributed to the strong electrostatic attraction between Na⁺ and Cl⁻ ions in its crystal lattice. In contrast, sucrose molecules are held together by weaker intermolecular forces, including hydrogen bonds, resulting in a significantly lower melting point. This example clearly demonstrates the impact of bonding mechanisms on melting points.
6.2. MgO vs. H₂O: The Role of Charge and Hydrogen Bonding
Another illustrative example is the comparison of magnesium oxide (MgO) and water (H₂O). MgO, with its divalent ions (Mg²⁺ and O²⁻), has an exceptionally high melting point of 2852°C (5166°F), reflecting the strong electrostatic interactions within its crystal lattice. Water, a covalent compound with hydrogen bonds, has a melting point of 0°C (32°F). Despite the presence of hydrogen bonds, the intermolecular forces in water are much weaker than the ionic bonds in MgO, leading to a substantial difference in melting points. This comparison highlights the dominant role of ionic charge and lattice energy in determining the thermal stability of ionic compounds.
7. Applications and Implications: Why Melting Points Matter
7.1. Materials Science: Designing High-Temperature Materials
The high melting points of ionic compounds are crucial in materials science, particularly in the design of high-temperature materials. Ceramics, which are often ionic compounds such as oxides, nitrides, and carbides, are used in applications requiring high thermal stability, such as furnace linings, aerospace components, and cutting tools. The ability of these materials to withstand extreme temperatures without melting or degrading is essential for their functionality. Understanding the relationship between ionic bonding and melting points allows materials scientists to develop new compounds with tailored thermal properties for specific applications.
7.2. Chemical Engineering: Optimizing Reaction Conditions
In chemical engineering, melting points play a vital role in optimizing reaction conditions and separation processes. The choice of solvents, catalysts, and reaction temperatures often depends on the melting points of the involved compounds. For instance, reactions involving solid reactants may require higher temperatures to ensure proper mixing and reaction rates. Similarly, separation techniques such as distillation and crystallization rely on differences in melting points and boiling points to isolate desired products. Knowledge of melting points enables chemical engineers to design efficient and cost-effective processes.
7.3. Pharmaceutical Development: Formulating Stable Drug Compounds
In pharmaceutical development, the melting points of drug compounds and excipients are critical for formulating stable and effective medications. The melting point of a drug can affect its solubility, bioavailability, and stability during storage. Excipients, which are inactive ingredients added to drug formulations, must have compatible melting points to ensure uniform mixing and prevent phase separation. Understanding the thermal properties of pharmaceutical compounds is essential for developing safe and reliable drug products.
8. Exceptions and Anomalies: When Predictions Don’t Hold True
8.1. Covalent Network Solids: Exceptionally High Melting Points
While covalent compounds generally have lower melting points than ionic compounds, there are exceptions, notably covalent network solids. These materials, such as diamond and silicon dioxide (quartz), consist of atoms connected by covalent bonds in an extended network, forming a giant molecule. The strong covalent bonds throughout the structure result in exceptionally high melting points. Diamond, for example, has a melting point of over 3550°C (6422°F), comparable to many ionic compounds. Similarly, silicon dioxide has a melting point of 1713°C (3115°F). These materials deviate from the typical behavior of covalent compounds due to their unique structural arrangements.
8.2. Ionic Liquids: Low Melting Points Despite Ionic Bonds
Conversely, some ionic compounds, known as ionic liquids, have surprisingly low melting points, often below 100°C (212°F). These compounds typically consist of bulky, asymmetric ions that disrupt the crystal lattice structure, reducing the electrostatic attractions and lowering the melting point. Ionic liquids have gained considerable attention as environmentally friendly solvents and electrolytes due to their unique properties. Examples include 1-ethyl-3-methylimidazolium ethyl sulfate ([EMIM][EtSO₄]) and other salts with large organic cations. The unusual behavior of ionic liquids highlights the complexity of predicting melting points based solely on bonding type.
9. Advanced Techniques for Determining Melting Points
9.1. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) is a widely used technique for accurately determining the melting points of both ionic and covalent compounds. DSC measures the heat flow associated with phase transitions, such as melting, as a function of temperature. By monitoring the heat absorbed or released during the melting process, DSC can precisely identify the melting point and provide information about the purity and thermal stability of the sample. This technique is particularly useful for characterizing complex materials and identifying phase transitions in mixtures.
9.2. X-Ray Diffraction (XRD)
X-ray diffraction (XRD) is another powerful technique for studying the structure and thermal behavior of crystalline materials. XRD involves bombarding a sample with X-rays and analyzing the diffraction pattern produced by the interaction of the X-rays with the crystal lattice. By analyzing the diffraction pattern at different temperatures, XRD can provide information about the changes in crystal structure associated with melting and other phase transitions. This technique is invaluable for understanding the relationship between crystal structure and thermal properties.
10. Conclusion: Ionic Compounds and High Melting Points
In summary, ionic compounds generally exhibit higher melting points compared to covalent compounds due to the strong electrostatic forces between ions in their crystal lattices. Lattice energy, determined by the charge and size of ions, plays a crucial role in dictating the thermal stability of ionic compounds. Covalent compounds, held together by weaker intermolecular forces, typically have lower melting points. However, exceptions such as covalent network solids and ionic liquids highlight the complexity of predicting melting points based solely on bonding type. Understanding the factors influencing melting points is essential in various fields, including materials science, chemical engineering, and pharmaceutical development. By leveraging advanced techniques such as DSC and XRD, researchers can accurately determine and characterize the thermal properties of materials, enabling the design of new compounds with tailored functionalities.
COMPARE.EDU.VN offers extensive comparisons and detailed analyses to help you make informed decisions in your scientific and industrial endeavors. Visit COMPARE.EDU.VN to explore a wealth of resources and enhance your understanding of material properties and chemical behaviors. For further inquiries, please contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or reach us via WhatsApp at +1 (626) 555-9090.
11. Frequently Asked Questions (FAQ)
11.1. Why do ionic compounds dissolve in water?
Ionic compounds dissolve in water because water is a polar solvent. The partially positive hydrogen atoms and partially negative oxygen atoms in water molecules can interact with the positive and negative ions in the ionic compound, respectively. This interaction, known as solvation, helps to overcome the electrostatic forces holding the ions together in the crystal lattice, allowing the ions to disperse throughout the water.
11.2. Are all ionic compounds soluble in water?
No, not all ionic compounds are soluble in water. The solubility of an ionic compound depends on the balance between the lattice energy of the compound and the hydration energy of the ions. If the hydration energy is greater than the lattice energy, the compound is soluble. Conversely, if the lattice energy is greater, the compound is insoluble. General solubility rules can help predict whether a particular ionic compound is likely to be soluble.
11.3. What are the general properties of covalent compounds?
Covalent compounds typically have lower melting and boiling points compared to ionic compounds. They can exist as solids, liquids, or gases at room temperature. Covalent compounds generally do not conduct electricity because they do not contain free mobile ions or electrons. Many covalent compounds are soluble in nonpolar solvents but insoluble in water.
11.4. How does hydrogen bonding affect the properties of covalent compounds?
Hydrogen bonding is a particularly strong type of intermolecular force that significantly affects the properties of covalent compounds. Compounds with hydrogen bonds, such as water and alcohols, have higher melting and boiling points compared to similar compounds without hydrogen bonds. Hydrogen bonding also influences the solubility, viscosity, and surface tension of these compounds.
11.5. What is lattice energy, and how does it influence the properties of ionic compounds?
Lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. It is a measure of the strength of the ionic bonds in the crystal lattice. Higher lattice energy indicates stronger ionic bonds and, consequently, higher melting points, boiling points, and hardness. Lattice energy is directly proportional to the charge of the ions and inversely proportional to the distance between them.
11.6. Can covalent compounds conduct electricity?
Generally, covalent compounds do not conduct electricity because they do not contain free mobile ions or electrons. However, some covalent compounds can become conductive under certain conditions. For example, graphite, a form of carbon, has a layered structure with delocalized electrons that can move freely, allowing it to conduct electricity. Additionally, some covalent compounds can be doped with impurities to increase their conductivity.
11.7. How do intermolecular forces differ from intramolecular forces?
Intermolecular forces are attractive forces or interactions between different molecules in a sample of a substance. These forces include London dispersion forces, dipole-dipole interactions, and hydrogen bonds. Intramolecular forces, on the other hand, are the forces that hold atoms together within a molecule, such as covalent bonds and ionic bonds. Intermolecular forces are generally weaker than intramolecular forces and influence the physical properties of substances, while intramolecular forces determine the chemical properties.
11.8. What are some real-world applications of ionic and covalent compounds?
Ionic and covalent compounds have a wide range of real-world applications. Ionic compounds are used in ceramics, fertilizers, de-icing salts, and antacids. Covalent compounds are used in plastics, pharmaceuticals, fuels, and solvents. The specific properties of each compound make them suitable for different applications.
11.9. How do the properties of ionic liquids differ from those of traditional ionic compounds?
Ionic liquids have lower melting points, higher thermal stability, negligible vapor pressure, and good electrical conductivity compared to traditional ionic compounds. They are often used as environmentally friendly solvents and electrolytes in various applications, including chemical reactions, electrochemical devices, and separation processes. The unique properties of ionic liquids arise from the bulky, asymmetric ions that disrupt the crystal lattice structure.
11.10. What role does electronegativity play in determining the type of bond formed between two atoms?
Electronegativity is a measure of the ability of an atom to attract electrons in a chemical bond. The difference in electronegativity between two atoms determines the type of bond formed between them. If the electronegativity difference is large (typically greater than 1.7), an ionic bond is formed. If the electronegativity difference is small (typically less than 0.4), a nonpolar covalent bond is formed. If the electronegativity difference is intermediate (between 0.4 and 1.7), a polar covalent bond is formed.
12. Call to Action
Are you struggling to compare different materials and compounds for your projects or studies? Visit COMPARE.EDU.VN today to access detailed comparisons, expert analyses, and comprehensive data that will help you make informed decisions. Whether you’re in materials science, chemical engineering, or any field requiring precise material selection, COMPARE.EDU.VN is your go-to resource for objective and thorough comparisons. Don’t make decisions in the dark—empower yourself with the knowledge you need to succeed. Head over to compare.edu.vn now and start comparing! Our dedicated team at 333 Comparison Plaza, Choice City, CA 90210, United States, is also available via WhatsApp at +1 (626) 555-9090 to assist you with any specific inquiries.