Molecular and ionic compounds exhibit distinct physical properties, largely dictated by the nature of their chemical bonds. Compared With Solid Ionic Compounds Solid Molecular Compounds Generally display significant differences in melting points, boiling points, electrical conductivity, and solubility. This contrast arises from the fundamental variations in their bonding and intermolecular forces.
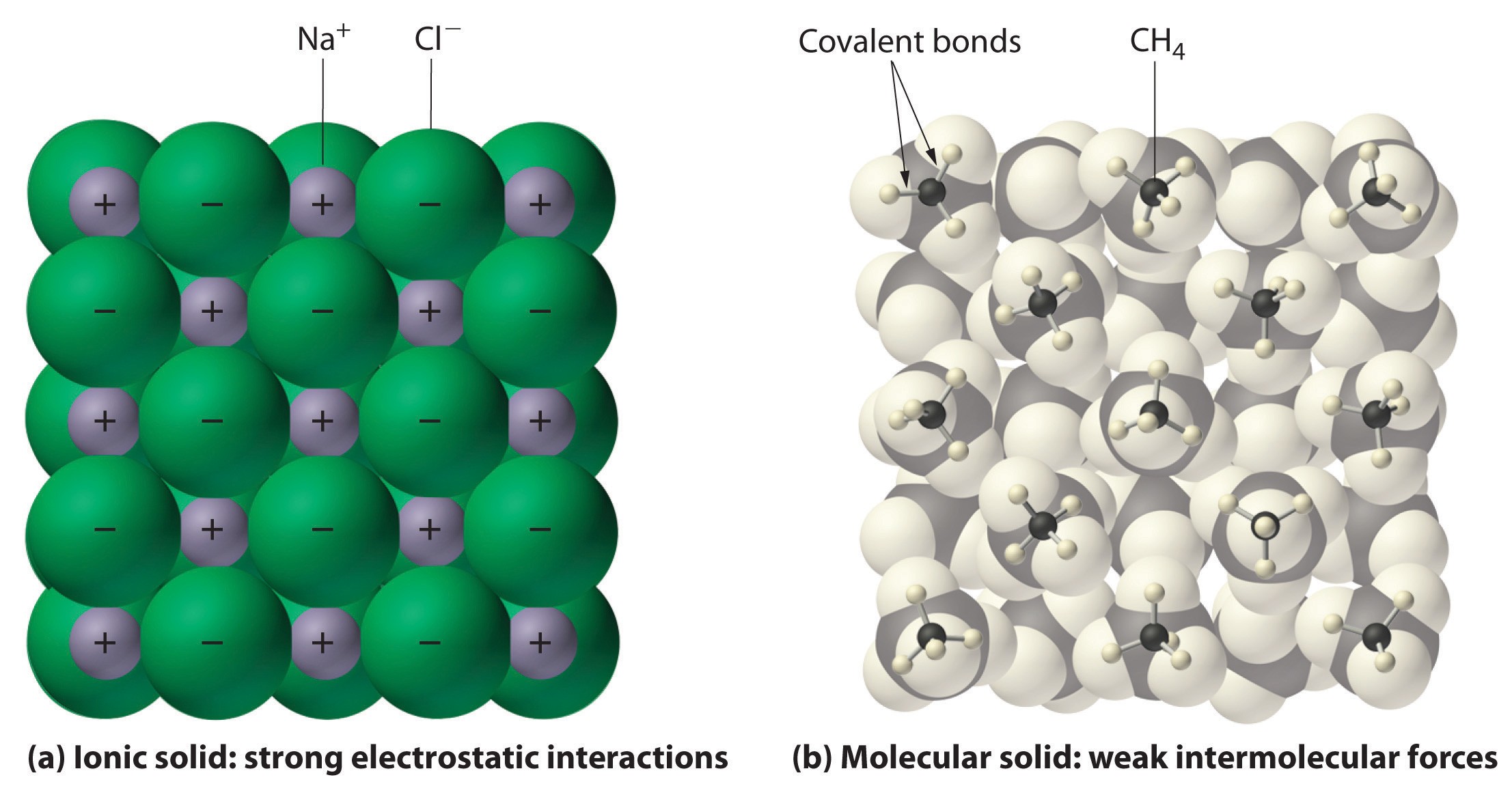 Visual comparison of (a) ionic solid crystal lattice structure with strong electrostatic interactions in Sodium Chloride (NaCl) and (b) molecular solid structure of Methane (CH4) with weak intermolecular forces.
Visual comparison of (a) ionic solid crystal lattice structure with strong electrostatic interactions in Sodium Chloride (NaCl) and (b) molecular solid structure of Methane (CH4) with weak intermolecular forces.
Bonding and Structure Differences
Ionic compounds, typically formed between metals and nonmetals, are characterized by ionic bonds. These bonds result from the transfer of electrons, creating positively charged ions (cations) and negatively charged ions (anions). In the solid state, ionic compounds form a crystal lattice structure. This lattice is a repeating three-dimensional array where each ion is surrounded by ions of opposite charge. The electrostatic attractions between these oppositely charged ions are strong and extend throughout the entire crystal. Sodium chloride (NaCl), or common table salt, is a classic example of an ionic solid.
Molecular compounds, on the other hand, are primarily formed between nonmetals and are held together by covalent bonds. Covalent bonds involve the sharing of electron pairs between atoms. In solid molecular compounds, individual molecules are held together by weaker intermolecular forces. These forces can be dipole-dipole interactions, London dispersion forces, or hydrogen bonds, all of which are significantly weaker than ionic bonds. Solid methane (CH4), where methane molecules are held together by weak London dispersion forces, exemplifies a molecular solid.
Melting and Boiling Points
One of the most striking differences between solid ionic and molecular compounds lies in their melting and boiling points. Solid ionic compounds generally possess high melting and boiling points. For instance, sodium chloride melts at a substantial 801 °C and boils at 1413 °C. This high energy requirement is due to the need to overcome the strong ionic bonds throughout the crystal lattice to transition into liquid or gaseous states.
Solid molecular compounds generally exhibit much lower melting and boiling points. Water (H2O), a molecular compound, melts at 0 °C and boils at 100 °C, a stark contrast to sodium chloride. The relatively low energy needed for phase changes in molecular compounds is because only the weak intermolecular forces between molecules need to be disrupted, not the strong covalent bonds within the molecules themselves.
Electrical Conductivity
Electrical conductivity also distinguishes solid ionic compounds from solid molecular compounds. In their solid form, ionic compounds are poor conductors of electricity. This is because the ions are fixed in the crystal lattice and are not free to move and carry an electrical charge. However, when ionic compounds are melted or dissolved in water, they become excellent conductors. In the molten or aqueous state, the ions are mobile and can facilitate the flow of electric current.
Molecular compounds, whether solid, liquid, or gaseous, are generally poor electrical conductors. Since they are composed of neutral molecules and lack freely moving charged particles like ions or electrons, they cannot effectively conduct electricity.
Water Solubility
The solubility in water varies significantly between these compound types. Solid ionic compounds are often highly soluble in water. Water, being a polar solvent, can effectively weaken the ionic bonds in the crystal lattice and surround the individual ions, leading to dissolution.
The water solubility of solid molecular compounds is more variable and depends on the polarity of the molecules and the intermolecular forces they can form with water. Polar molecular compounds like sugar can dissolve in water due to hydrogen bonding, while nonpolar molecular compounds like oil are largely insoluble because they cannot form favorable interactions with water.
Covalent Network Solids: An Exception
It’s important to note an exception within molecular compounds: covalent network solids. These are compounds where atoms are interconnected by covalent bonds in a continuous network. Diamond, a form of carbon, is a prime example. In diamond, each carbon atom is covalently bonded to four other carbon atoms, forming a giant network.
Covalent network solids like diamond have extremely high melting points, even higher than many ionic compounds. Melting them requires breaking the strong covalent bonds throughout the network, not just weak intermolecular forces. Diamond, in fact, sublimes (transitions directly from solid to gas) at temperatures above 3500 °C rather than melting.
Conclusion
In summary, compared with solid ionic compounds solid molecular compounds generally present lower melting and boiling points, and are poor electrical conductors in all states. These differences stem from the strong ionic bonds in ionic compounds versus the weaker intermolecular forces in molecular compounds. While covalent network solids represent a special case within molecular compounds with exceptionally high melting points due to their extensive covalent bonding, the general trend holds true for most comparisons between solid ionic and molecular substances.