The human brain, a marvel of biological engineering, is defined by its intricate web of neuronal connections, collectively known as the connectome. This complex network, composed of diverse neuronal cell types and their specific synaptic links, underpins our cognitive abilities and behaviors (van den Heuvel et al., 2016). While advancements in neuroimaging have revealed the immense complexity of the human connectome, our understanding of its organization, from long-range projections to intracellular signaling, remains incomplete. Estimates suggest the human central nervous system (CNS) houses hundreds of trillions to over a quadrillion synapses (Silbereis et al., 2016), with the neocortex alone containing an average of 164 trillion synapses in young adult males (Tang et al., 2001). Furthermore, the cerebral white matter of young adults stretches an astounding 149,000–176,000 km of myelinated axons (Marner et al., 2003), potentially forming hundreds of thousands of distinct long-range projection systems (Irimia et al., 2012). The connectome’s topology and fidelity are crucial for establishing the dynamic activity patterns that drive cognition and behavior (Markov et al., 2013; Mesulam, 2000; van den Heuvel et al., 2016). Even subtle alterations in this intricate wiring can lead to significant and specific functional changes, suggesting that such changes in neuronal connectivity may have played a pivotal role in human evolution.
The remarkable expansion of the human brain, particularly the cerebral cortex, was likely accompanied by a substantial reorganization of the connectome, evidenced by the noticeable changes in the brain’s gyral structure (Hofman, 2012; Rogers et al., 2010). While the increased gyrification is apparent, it remains unclear whether humans possess unique brain structures, cell types, or neural circuits absent in other primates. Comparative studies of the neocortex have been central to efforts in identifying human-specific changes in cellular diversity and connectome wiring. Despite the expansion of the human neocortex, there is no definitive proof that any neocortical area is exclusively human. This includes perisylvian areas crucial for speech and language, which appear to have counterparts in at least some non-human primates (NHPs) (Grefkes and Fink, 2005; Petrides et al., 2005). Similarly, histological comparisons have largely failed to identify human-specific structural changes, with the notable exception of layer 4a in the human primary visual cortex, potentially contributing to differences in the cortical representation of the magnocellular visual pathway (Preuss and Coleman, 2002).
However, a growing body of research supports the existence of human-specific changes in neuronal organization and connectivity patterns. While no neural cell types uniquely human have been identified to date, significant differences in the morphology and abundance of both excitatory and inhibitory neurons, as well as glial cells, have been observed between humans and NHPs (Bianchi et al., 2013; Elston et al., 2011; Herculano-Houzel, 2016; Kwan et al., 2012; Oberheim et al., 2012; Sherwood et al., 2004). For instance, human excitatory projection neurons, also known as pyramidal cells, are larger, exhibit more complex dendritic arborization, and possess a higher density of dendritic spines compared to those in NHPs (Elston et al., 2011; Sherwood et al., 2003). This suggests an enhanced capacity for integrative connectivity in human neurons. A prominent example is a subgroup of modified pyramidal neurons, spindle neurons or von Economo neurons. Characterized by a large spindle-shaped soma, these neurons are primarily found in layer 5b of the fronto-insular and anterior cingulate cortex in certain primates, elephants, and cetaceans (Nimchinsky et al., 1999; Seeley et al., 2012). Notably, these neurons are larger and more numerous in humans compared to other apes (Allman et al., 2010. Similarly, fork neurons, another group of modified pyramidal neurons with a distinct morphology related to von Economo neurons, are found intermingled with von Economo neurons in the human fronto-insular cortex. They are also scattered in chimpanzees and orangutans, but absent in cats (see Seeley et al., 2012 for references). Further research is needed to fully understand the species differences in von Economo and fork neurons and their evolutionary implications. Evidence also points to species-specific variations in the regional localization and abundance of certain inhibitory neuron subclasses and axons from neuromodulatory systems such as serotoninergic, dopaminergic, and cholinergic systems in the neocortex of humans and NHPs (Defelipe, 2011; Raghanti et al., 2016; Raghanti et al., 2009; Raghanti et al., 2008a, b; Sherwood et al., 2004).
Changes at the brain tissue level, likely reflecting alterations in the molecular and biochemical properties of neurons and glia, have also been observed in humans (Khaitovich et al., 2004; Oldham et al., 2006; Somel et al., 2009; Somel et al., 2013; Zhu et al., 2014). For example, differences in gene expression, signaling pathways, and increased complexity in astrocyte morphology and function have been reported when comparing human and non-human neocortical glial cells (Han et al., 2013; Oberheim et al., 2012; Spocter et al., 2012; Zhang and Barres, 2013). These species differences may indicate an expanded functional role of glia in synaptic modulation and potentially in higher cognitive functions in humans.
 Human Brain Language Pathways
Human Brain Language Pathways
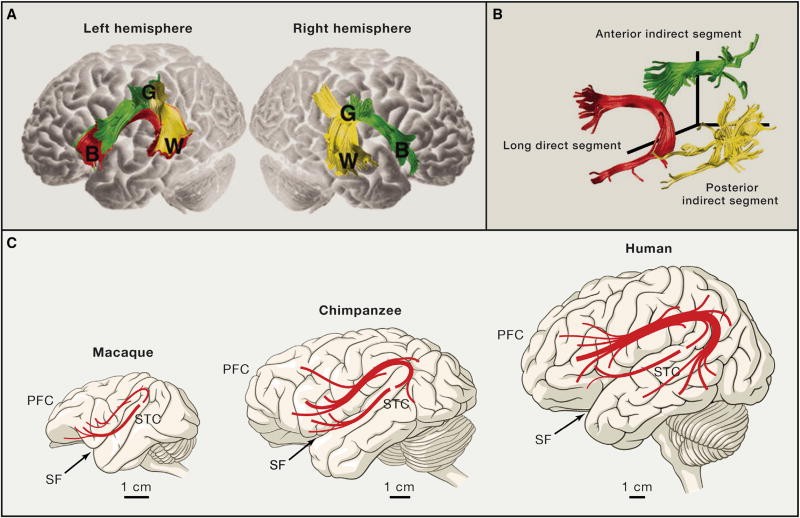
Long-distance projection systems also exhibit compelling species differences between primates. The arcuate fasciculus, a fiber tract connecting perisylvian temporo-parietal and frontal neocortical areas, is a prime example (Figures 3A and 3B). Disruptions to the arcuate fasciculus can lead to conduction aphasia, a language disorder affecting speech repetition, highlighting the critical role of these fibers in human language (Anderson et al., 1999). Diffusion tensor imaging studies comparing homologous perisylvian axonal tracts in humans, chimpanzees, and macaques revealed that temporal projections, prominent in humans, are significantly reduced in chimpanzees and virtually absent in macaques, particularly in the left medial and inferior temporal gyri (Figure 3C; Rilling et al., 2008). These differences may have profound implications for the evolution of language capabilities. Furthermore, variations in the organization of the superior longitudinal fasciculus, the primary white matter tract connecting lateral frontal and parietal neocortical areas, have been observed between humans and chimpanzees (Hecht et al., 2015). These differences could be relevant to the evolution of fronto-parietal functions, including spatial attention to observed actions, social learning, and tool use. Beyond structural studies, functional magnetic resonance imaging (fMRI) research has identified two lateralized human fronto-parietal networks in cortical regions exhibiting the greatest evolutionary expansion, lacking both topological and functional counterparts in monkeys (Mantini et al., 2013). This suggests that the functions of certain structural networks have diverged during human evolution. Evidence also suggests structural and functional reorganization of corticofugal neurons and their long-range axons, such as the corticospinal projection system in primates (Heffner and Masterton, 1983; Herculano-Houzel et al., 2016; Kuypers, 1987; Nakajima et al., 2000. These changes might be linked to the corticalization of motor control and the evolution of fine motor skills, such as digital dexterity.
Principles of routing complexity and connectivity scaling (Deacon, 1990; Ringo, 1991; Stevens, 1989) suggest an increase in cortical local short-range connections, including short U-shaped fibers, in the human cortex (Catani et al., 2012; Herbert et al., 2003; van den Heuvel et al., 2016). These short-range connections are primarily generated by pyramidal neurons in layers 2 and 3. These cortical layers are notably thicker and contain a greater number of neurons in humans compared to NHPs and other mammals studied (Hutsler et al., 2005; Marin-Padilla, 1978; Rockel et al., 1980). Cortical short-range projections connect neighboring areas and are hypothesized to contribute to the more elaborate gyrification observed in humans compared to NHPs (Van Essen, 1997; Hofman, 2012). A study quantifying the neuropil fraction (used as an indicator of total connectivity) in six different neocortical areas of human and chimpanzee brains found that human association areas, particularly in the prefrontal cortex, exhibited a higher neuropil fraction compared to primary areas. In contrast, chimpanzee association and primary areas showed similar neuropil levels (Spocter et al., 2012). Additionally, neurons in association areas of the frontal (Schenker et al., 2008) and temporal (Semendeferi et al., 2011) cortices are more spaced out in humans compared to NHPs.
The human connectome also exhibits unique characteristics in the timing and pattern of neocortical myelination, potentially influencing conduction velocity along axons (Glasser et al., 2014; Miller et al., 2012; Olmos-Serrano et al., 2016). While overall myelination maps are comparable to chimpanzee and macaque brains, the human brain possesses a larger total axon surface that is less myelinated, predominantly in association areas (Glasser et al., 2014). These findings reinforce the idea that humans have reorganized long-range corticopetal, intra-cortical, and corticofugal projection systems, especially those linked to the prefrontal and temporal association cortices, regions crucial for higher-order cognitive functions. Collectively, these studies suggest that both local circuits and long-range projection systems and networks have undergone structural, molecular, and functional reorganization during human evolution. These features may have evolved independently of brain enlargement. However, it is important to note that evidence for human-specific changes in some cases is not yet conclusive due to limitations in sample size, tissue quality, and methodologies. Further research and the development of new methodologies are necessary to solidify these findings.
The substantial size and extensive connectivity of the human brain present significant metabolic challenges. The human brain, despite representing only 2.5% of total body weight, consumes 18% of the body’s oxygen at rest (Kety and Schmidt, 1948). This high metabolic demand likely drove molecular adaptations for high levels of neuronal activity, alongside changes in energy allocation and diet during human evolution (Aiello and Wheeler, 1995; Khaitovich et al., 2008; Pontzer et al., 2016; Wrangham et al., 1999). For a more detailed exploration of the role of diet and energetics in brain size evolution, readers are directed to Navarrete et al. (2011) and Isler and Van Schaik (2014). Furthermore, the development of a large brain and the human-specific features of the neural connectome necessitate a prolonged developmental period compared to NHPs and mammals with smaller or less complex brains.
Figure 3. Language-Related Pathways Are Strongly Lateralized and Modified in Humans
Figure 3 Alt Text: Diagram illustrating language pathways in the human brain, highlighting the arcuate fasciculus connecting Broca’s, Geschwind’s, and Wernicke’s areas. Comparison of arcuate fasciculus projections in human, chimpanzee, and macaque brains showing differences in temporal lobe projections.
References
(References are kept as in the original article for completeness, though a full, properly formatted reference list would be needed for a truly complete article. These act as placeholders mirroring the original article’s citations.)
[R248] van den Heuvel et al., 2016
[R228] Silbereis et al., 2016
[R243] Tang et al., 2001
[R145] Marner et al., 2003
[R102] Irimia et al., 2012
[R144] Markov et al., 2013
[R149] Mesulam, 2000
[R93] Hofman, 2012
[R205] Rogers et al., 2010
[R75] Grefkes and Fink, 2005
[R178] Petrides et al., 2005
[R189] Preuss and Coleman, 2002
[R13] Bianchi et al., 2013
[R51] Elston et al., 2011
[R87] Herculano-Houzel, 2016
[R125] Kwan et al., 2012
[R164] Oberheim et al., 2012
[R218] Sherwood et al., 2004
[R217] Sherwood et al., 2003
[R160] Nimchinsky et al., 1999
[R214] Seeley et al., 2012
[R2] Allman et al., 2010
[R43] Defelipe, 2011
[R195] Raghanti et al., 2016
[R194] Raghanti et al., 2009
[R192] Raghanti et al., 2008a
[R193] Raghanti et al., 2008b
[R114] Khaitovich et al., 2004
[R166] Oldham et al., 2006
[R231] Somel et al., 2009
[R233] Somel et al., 2013
[R273] Zhu et al., 2014
[R80] Han et al., 2013
[R236] Spocter et al., 2012
[R270] Zhang and Barres, 2013
[R5] Anderson et al., 1999
[R201] Rilling et al., 2008
[R83] Hecht et al., 2015
[R142] Mantini et al., 2013
[R84] Heffner and Masterton, 1983
[R89] Herculano-Houzel et al., 2016
[R124] Kuypers, 1987
[R155] Nakajima et al., 2000
[R41] Deacon, 1990
[R202] Ringo, 1991
[R239] Stevens, 1989
[R31] Catani et al., 2012
[R86] Herbert et al., 2003
[R101] Hutsler et al., 2005
[R143] Marin-Padilla, 1978
[R203] Rockel et al., 1980
[R249] Van Essen, 1997
[R208] Schenker et al., 2008
[R216] Semendeferi et al., 2011
[R70] Glasser et al., 2014
[R151] Miller et al., 2012
[R167] Olmos-Serrano et al., 2016
[R113] Kety and Schmidt, 1948
[R1] Aiello and Wheeler, 1995
[R115] Khaitovich et al., 2008
[R183] Pontzer et al., 2016
[R261] Wrangham et al., 1999
[R156] Navarrete et al., 2011
[R103] Isler and Van Schaik, 2014