Comparing volumes accurately involves understanding the principles of volume measurement, choosing the right tools, and applying appropriate techniques. COMPARE.EDU.VN helps you navigate these complexities, ensuring you get the most accurate results. This guide dives deep into various methods and software solutions to help you achieve precise volume comparisons, incorporating relative density changes and overcoming positioning discrepancies.
1. What Are The Fundamental Concepts In Comparing Volumes?
The fundamental concepts include understanding volume units (cm³, mm³, mL), voxel values, and the importance of accurate registration. Precise volume comparison hinges on grasping these core elements, enabling reliable assessment and analysis.
1.1 Understanding Volume Units (cm³, mm³, mL)
Volume units are essential for quantifying three-dimensional space. Cubic centimeters (cm³) are commonly used for moderate-sized objects, while cubic millimeters (mm³) are suitable for smaller, more precise measurements. Milliliters (mL) are often used interchangeably with cm³ in many contexts, as 1 mL is equivalent to 1 cm³.
- Cubic Centimeters (cm³): A cubic centimeter is the volume of a cube with sides of 1 cm each. It is used in various applications, including medical imaging and material science.
- Cubic Millimeters (mm³): A cubic millimeter is a smaller unit, representing the volume of a cube with sides of 1 mm each. It’s valuable for detailed measurements where precision is crucial.
- Milliliters (mL): A milliliter is a unit of volume often used for liquids but is also directly equivalent to 1 cm³. This unit is commonly used in medical and scientific fields.
Understanding these units and their conversions is crucial for accurate volume comparison.
1.2 What Is The Significance Of Voxel Values In Volume Comparison?
Voxel values represent the density or intensity of a three-dimensional pixel in medical imaging data like CBCT scans. Comparing voxel values helps assess changes in relative density, which can indicate bone growth, tissue changes, or other important clinical information.
- Definition of Voxels: Voxels are the three-dimensional equivalent of pixels, representing a single point in a 3D space.
- Density and Intensity: Voxel values typically correspond to the density of the tissue or material at that location. In CBCT scans, higher voxel values might indicate denser bone.
- Relative Density Changes: By comparing voxel values between pre- and post-operative scans, you can quantify changes in density within a specific region of interest (ROI).
- Clinical Applications: This is particularly useful in monitoring bone graft integration, assessing tumor growth, or evaluating the effectiveness of treatments.
1.3 Why Is Accurate Registration Essential For Volume Comparison?
Accurate registration aligns pre- and post-operative scans to correct for positioning discrepancies. Without precise registration, comparing volumes and voxel values can lead to significant errors. Registration ensures that you are quantifying changes in the same anatomical region across different scans.
- Correcting Positioning Discrepancies: Patients may not be positioned identically in each scan, leading to misalignments.
- Ensuring Anatomical Correspondence: Registration algorithms align the scans based on anatomical landmarks, ensuring that the same ROI is being compared.
- Minimizing Errors: Accurate registration minimizes errors in volume and voxel value comparisons, providing reliable results.
- Techniques for Registration: Common registration techniques include rigid registration, which corrects for translations and rotations, and non-rigid registration, which can account for more complex deformations.
2. What Are The Tools And Software For Comparing Volumes?
Various tools and software are available for comparing volumes, including 3D Slicer, Autodesk Meshmixer, and CloudCompare. Each tool has its strengths and weaknesses, making it important to choose the right one for your specific needs.
2.1 How To Use 3D Slicer For Volume Comparison?
3D Slicer is a powerful, open-source software platform for medical image analysis. It can be used for segmentation, registration, and volume quantification. Its modular design allows for a customizable workflow, making it suitable for a variety of research and clinical applications.
- Segmentation: 3D Slicer allows you to define regions of interest (ROIs) using manual or semi-automatic segmentation tools. You can create segments to represent specific anatomical structures.
- Registration: The registration module in 3D Slicer can align pre- and post-operative scans. Options include rigid, affine, and non-rigid registration algorithms.
- Volume Quantification: The “Segment Statistics” module provides detailed information about each segment, including volume, surface area, and voxel statistics.
- Change Tracking: The ChangeTracker module is useful for quantifying changes between registered scans. It calculates volume differences and voxel value changes.
- Workflow Example:
- Import DICOM data: Load the pre- and post-operative CBCT scans into 3D Slicer.
- Segment the ROI: Use segmentation tools to define the region of interest (e.g., bone graft area) in both scans.
- Register the scans: Use the registration module to align the post-operative scan to the pre-operative scan.
- Quantify volume changes: Use the ChangeTracker module or Segment Statistics to calculate volume differences and voxel value changes.
 3CEFCEE78CCF4F9B873E24E85600BBAB.jpg showing a 3D Slicer interface with segmentation and registration tools
3CEFCEE78CCF4F9B873E24E85600BBAB.jpg showing a 3D Slicer interface with segmentation and registration tools
2.2 What Is Autodesk Meshmixer And How Does It Assist Volume Comparison?
Autodesk Meshmixer is a free software tool for working with 3D meshes. It can be used to create solid models from segmentations, smooth surfaces, and prepare models for 3D printing or further analysis.
- STL File Creation: Meshmixer can import STL files exported from 3D Slicer or other segmentation software.
- Model Solidification: It allows you to create solid, watertight models from segmented data.
- Surface Smoothing: Meshmixer can smooth the surfaces of 3D models, improving their appearance and accuracy.
- Preparation for Registration: The software is valuable for preparing models for registration in other software like CloudCompare.
- Workflow Integration: While Meshmixer doesn’t directly compare volumes, it facilitates the creation of accurate 3D models that can be used in subsequent comparison steps.
2.3 How Does CloudCompare Contribute To Volume Comparison?
CloudCompare is an open-source software for 3D point cloud and mesh processing. It is often used for registering 3D models and calculating distances between them.
- Model Registration: CloudCompare can register pre- and post-operative models using algorithms like Iterative Closest Point (ICP).
- Distance Calculation: It calculates the distances between two registered models, providing insights into changes in shape and volume.
- Volume Calculation: CloudCompare can calculate the volume of 3D models.
- Change Detection: The software can identify areas of growth or shrinkage between two time points.
- Workflow Integration:
- Export Models: Export the pre- and post-operative models from 3D Slicer or Meshmixer as STL files.
- Import Models: Import the STL files into CloudCompare.
- Register Models: Use the registration tools to align the models.
- Calculate Volume Differences: Calculate the volume of each model and determine the volume difference.
3. What Methods Can Be Used For Comparing Volumes?
Several methods can be used for comparing volumes, including manual segmentation, semi-automatic segmentation, and change tracker modules. Each method has its advantages and disadvantages, making it important to select the right one for your specific needs.
3.1 Manual Segmentation: Advantages And Disadvantages
Manual segmentation involves manually outlining the region of interest (ROI) on each slice of a medical image. It is a time-consuming process but can be highly accurate if performed carefully.
- Advantages:
- High Accuracy: When performed meticulously, manual segmentation can provide very accurate results, especially in complex anatomical regions.
- Control: The user has complete control over the segmentation process, allowing for adjustments based on anatomical knowledge and clinical expertise.
- Adaptability: Manual segmentation can be adapted to a wide range of image types and anatomical structures.
- Disadvantages:
- Time-Consuming: It is a labor-intensive process that can take hours to complete, especially for large datasets.
- Subjectivity: Manual segmentation is subject to inter- and intra-observer variability, meaning that different users may produce different results.
- Expertise Required: It requires a high level of anatomical knowledge and clinical expertise.
3.2 How Does Semi-Automatic Segmentation Improve Volume Comparison?
Semi-automatic segmentation combines manual input with automated algorithms to improve efficiency and reduce subjectivity. Techniques like thresholding, region growing, and active contours can be used to assist the segmentation process.
- Thresholding:
- Process: Selects voxels based on their intensity values.
- Use Case: Useful for segmenting tissues with distinct density ranges (e.g., bone).
- Advantages: Quick and simple.
- Disadvantages: Can be sensitive to noise and variations in image intensity.
- Region Growing:
- Process: Starts from a seed point and adds neighboring voxels based on similarity criteria.
- Use Case: Suitable for segmenting connected regions with homogeneous properties.
- Advantages: Can handle some variations in image intensity.
- Disadvantages: Sensitive to the choice of seed point and similarity criteria.
- Active Contours (Snakes):
- Process: Deforms a curve or surface to fit the boundaries of the ROI.
- Use Case: Effective for segmenting complex shapes and structures.
- Advantages: Robust to noise and can handle complex shapes.
- Disadvantages: Requires careful initialization and parameter tuning.
- Advantages of Semi-Automatic Segmentation:
- Improved Efficiency: Reduces the time required for segmentation compared to manual methods.
- Reduced Subjectivity: Minimizes inter- and intra-observer variability.
- Increased Accuracy: Can provide more accurate results than manual segmentation in some cases.
3.3 Change Tracker Modules: Are They Reliable For Volume Comparison?
Change tracker modules are designed to quantify changes between registered scans. They automatically calculate volume differences and voxel value changes, providing a convenient way to assess treatment outcomes.
- Functionality:
- Registration: Automatically registers pre- and post-operative scans.
- Volume Difference Calculation: Calculates the difference in volume between the ROIs.
- Voxel Value Changes: Quantifies changes in voxel values within the ROIs.
- Reliability Factors:
- Registration Accuracy: The accuracy of the change tracker module depends on the quality of the registration algorithm.
- Segmentation Consistency: Consistent segmentation of the ROI in both scans is essential.
- Algorithm Validation: The algorithm should be validated on relevant datasets to ensure accuracy and reliability.
- Considerations:
- Module Limitations: Be aware of the limitations of the specific change tracker module being used.
- Clinical Validation: Validate the results of the change tracker module with clinical findings.
- Alternative Methods: Compare the results with alternative methods to ensure consistency.
4. How To Address Positioning Discrepancies In CBCT Scans?
Positioning discrepancies can significantly affect the accuracy of volume comparisons. Techniques such as rigid registration and iterative closest point (ICP) can help correct for these discrepancies.
4.1 What Is Rigid Registration And How Does It Work?
Rigid registration aligns two datasets by applying translations and rotations. It assumes that the objects being compared are rigid and do not undergo deformation.
- Process:
- Feature Extraction: Identifies corresponding anatomical landmarks in the pre- and post-operative scans.
- Transformation Estimation: Estimates the optimal translation and rotation parameters to align the landmarks.
- Transformation Application: Applies the estimated transformation to the post-operative scan.
- Use Cases:
- Correcting for simple positioning errors.
- Aligning datasets where the anatomical structures are rigid.
- Limitations:
- Cannot correct for deformations or non-rigid changes.
- May not be accurate if there are significant anatomical changes between the scans.
4.2 Iterative Closest Point (ICP): An Alternative For Registration
Iterative Closest Point (ICP) is an algorithm that iteratively aligns two 3D models by minimizing the distance between corresponding points.
- Process:
- Initial Alignment: Starts with an initial alignment of the two models.
- Point Correspondence: Identifies the closest point on the second model for each point on the first model.
- Transformation Estimation: Estimates the transformation that minimizes the distance between corresponding points.
- Transformation Application: Applies the estimated transformation to the second model.
- Iteration: Repeats steps 2-4 until the alignment converges.
- Use Cases:
- Aligning 3D models with complex shapes.
- Correcting for positioning errors in CBCT scans.
- Advantages:
- Can handle complex shapes and structures.
- Robust to noise and outliers.
- Disadvantages:
- Sensitive to the initial alignment.
- Can be computationally intensive.
4.3 When Should Non-Rigid Registration Be Considered?
Non-rigid registration aligns two datasets by allowing for local deformations. It is used when the objects being compared are not rigid and undergo significant changes in shape.
- Use Cases:
- Correcting for deformations due to soft tissue changes.
- Aligning datasets where there are significant anatomical changes between the scans.
- Techniques:
- B-Spline Registration: Uses B-spline curves to model the deformation field.
- Demons Registration: Based on the concept of optical flow and models the deformation field as a velocity field.
- Thin-Plate Spline Registration: Uses thin-plate splines to interpolate the deformation field.
- Considerations:
- Non-rigid registration is more computationally intensive than rigid registration.
- It requires careful parameter tuning to avoid over-fitting.
- The accuracy of non-rigid registration depends on the quality of the deformation model.
5. How To Quantify Volume Difference After Bone Grafting?
Quantifying volume difference after bone grafting involves segmenting the grafted area, registering pre- and post-operative scans, and calculating the volume change.
5.1 What Are The Key Steps In Quantifying Volume Change?
The key steps include accurate segmentation, precise registration, and appropriate volume calculation methods. Attention to detail in each step ensures reliable and meaningful results.
- Segmentation of Grafted Area:
- Manual Segmentation: Manually outline the grafted area on each slice of the CBCT scans.
- Semi-Automatic Segmentation: Use thresholding, region growing, or active contours to assist the segmentation process.
- Consistency: Ensure consistent segmentation criteria are applied to both pre- and post-operative scans.
- Registration of Pre- And Post-Operative Scans:
- Rigid Registration: Use rigid registration to correct for simple positioning errors.
- Non-Rigid Registration: Consider non-rigid registration if there are significant deformations.
- Accuracy: Ensure the registration is accurate to avoid errors in volume calculation.
- Volume Calculation:
- Segment Statistics: Use the Segment Statistics module in 3D Slicer to calculate the volume of the grafted area.
- ChangeTracker Module: Use the ChangeTracker module to quantify the volume difference between the scans.
- CloudCompare: Use CloudCompare to calculate the volume of the 3D models and determine the volume difference.
5.2 How To Deal With Artifacts And Noise In CBCT Images?
Artifacts and noise can affect the accuracy of volume comparisons. Techniques such as image filtering and artifact removal can help improve image quality.
- Image Filtering:
- Gaussian Filter: Smooths the image and reduces noise.
- Median Filter: Removes impulse noise and preserves edges.
- Anisotropic Diffusion Filter: Smooths the image while preserving important features.
- Artifact Removal:
- Metal Artifact Reduction (MAR): Reduces artifacts caused by metal implants.
- Beam Hardening Correction: Corrects for artifacts caused by the hardening of the X-ray beam.
- Software Tools:
- 3D Slicer: Offers various filtering and artifact removal tools.
- Dedicated MAR Software: Specialized software for metal artifact reduction.
- Considerations:
- Apply filters carefully to avoid blurring important details.
- Validate the effectiveness of artifact removal techniques.
5.3 What Are The Potential Sources Of Error And How To Minimize Them?
Potential sources of error include segmentation errors, registration errors, and image artifacts. Minimizing these errors requires careful attention to detail and the use of appropriate techniques.
- Segmentation Errors:
- Source: Inaccurate or inconsistent segmentation of the ROI.
- Minimization: Use semi-automatic segmentation techniques, train users, and validate segmentations.
- Registration Errors:
- Source: Misalignment of pre- and post-operative scans.
- Minimization: Use accurate registration algorithms, validate registration results, and consider non-rigid registration if necessary.
- Image Artifacts:
- Source: Artifacts caused by metal implants, beam hardening, or patient movement.
- Minimization: Use image filtering and artifact removal techniques.
- Volume Calculation Errors:
- Source: Errors in the volume calculation algorithm or software.
- Minimization: Validate the volume calculation results and use multiple software tools for comparison.
- General Tips:
- Calibration: Regularly calibrate the CBCT scanner.
- Training: Train users in proper scanning and analysis techniques.
- Validation: Validate results with clinical findings.
6. How To Measure Change Of Relative Density Using Voxel Values?
Measuring changes in relative density using voxel values involves comparing voxel intensities within the ROI between pre- and post-operative scans.
6.1 What Is The Relationship Between Voxel Value And Relative Density?
Voxel value is directly related to the density of the tissue or material at that location. Higher voxel values generally indicate denser tissue.
- CBCT Scans: In CBCT scans, voxel values represent the attenuation of X-rays as they pass through the tissue.
- Hounsfield Units (HU): Voxel values can be converted to Hounsfield Units (HU), which provide a standardized measure of tissue density. However, relative values can be used when scanner settings are consistent.
- Relative Density: By comparing voxel values between pre- and post-operative scans, you can assess changes in relative density within the ROI.
- Clinical Significance: Changes in relative density can indicate bone growth, tissue changes, or the effectiveness of treatments.
6.2 How To Normalize Voxel Values For Accurate Comparison?
Normalizing voxel values can help reduce the effects of variations in scanner settings and patient-specific factors.
- Methods for Normalization:
- Z-Score Normalization: Converts voxel values to a standard normal distribution with a mean of 0 and a standard deviation of 1.
- Min-Max Normalization: Scales voxel values to a range between 0 and 1.
- Histogram Matching: Adjusts the voxel values to match the distribution of a reference image.
- Software Tools:
- 3D Slicer: Offers various normalization tools.
- ImageJ: A widely used image processing software with normalization plugins.
- Considerations:
- Choose the appropriate normalization method for your specific application.
- Validate the effectiveness of the normalization.
6.3 What Statistical Methods Can Be Used To Compare Voxel Value Changes?
Statistical methods can be used to quantify and assess the significance of voxel value changes between pre- and post-operative scans.
- Statistical Tests:
- Paired T-Test: Compares the mean voxel values within the ROI between the two scans.
- Wilcoxon Signed-Rank Test: A non-parametric test that compares the median voxel values.
- Kolmogorov-Smirnov Test: Compares the distribution of voxel values between the two scans.
- Software Tools:
- R: A statistical programming language for data analysis.
- SPSS: A statistical software package.
- Considerations:
- Choose the appropriate statistical test for your data.
- Correct for multiple comparisons to avoid false positives.
- Report the statistical significance and effect size.
7. What Are The Best Practices For Ensuring Accuracy In Volume Comparison?
Ensuring accuracy in volume comparison requires careful attention to detail, proper technique, and validation of results.
7.1 Standardizing Scanning Protocols For Consistent Results
Standardizing scanning protocols helps minimize variability and improve the reliability of volume comparisons.
- Scanner Settings:
- Use the same CBCT scanner for all scans.
- Use consistent scanner settings (e.g., voltage, current, exposure time).
- Calibrate the scanner regularly.
- Patient Positioning:
- Use a consistent patient positioning protocol.
- Use immobilization devices to minimize movement.
- Image Reconstruction:
- Use the same reconstruction parameters for all scans.
- Use the same field of view.
- Documentation:
- Document all scanning parameters and protocols.
7.2 Validating Segmentation And Registration Results
Validating segmentation and registration results is essential for ensuring accuracy and reliability.
- Segmentation Validation:
- Visual Inspection: Visually inspect the segmentation results to ensure they are accurate.
- Inter-Observer Variability: Compare the segmentations performed by different users.
- Gold Standard: Compare the segmentations to a gold standard (e.g., manual segmentation by an expert).
- Registration Validation:
- Visual Inspection: Visually inspect the registered scans to ensure they are aligned correctly.
- Landmark-Based Validation: Measure the distance between corresponding landmarks in the registered scans.
- Target Registration Error (TRE): Calculate the TRE to quantify the accuracy of the registration.
7.3 Documenting The Entire Process For Reproducibility
Documenting the entire process is crucial for ensuring reproducibility and transparency.
- Documentation Elements:
- Scanning Protocols: Document all scanning parameters and protocols.
- Software Versions: Document the versions of all software used.
- Segmentation Methods: Document the segmentation methods and parameters used.
- Registration Methods: Document the registration methods and parameters used.
- Volume Calculation Methods: Document the volume calculation methods and parameters used.
- Statistical Analysis: Document the statistical methods used.
- Benefits of Documentation:
- Reproducibility: Allows others to reproduce the results.
- Transparency: Provides a clear and detailed record of the entire process.
- Quality Control: Helps identify and correct errors.
8. Case Studies: Practical Applications Of Volume Comparison
Examining case studies illustrates the practical applications of volume comparison in various clinical scenarios.
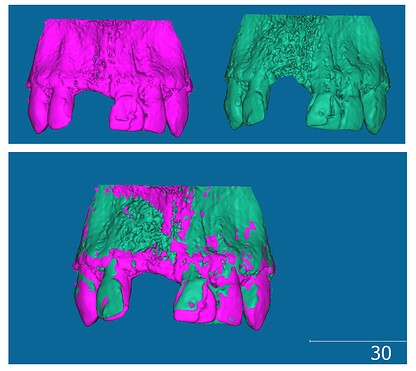
8.1 Monitoring Bone Graft Healing In Dental Implants
Volume comparison is valuable for monitoring bone graft healing around dental implants. By comparing pre- and post-operative CBCT scans, clinicians can assess the success of the graft and identify potential complications.
- Process:
- Segmentation: Segment the bone graft area in both pre- and post-operative scans.
- Registration: Register the scans to correct for positioning errors.
- Volume Calculation: Calculate the volume of the bone graft in both scans.
- Comparison: Compare the volumes to assess the amount of bone growth.
- Clinical Significance:
- Graft Success: Indicates whether the bone graft has successfully integrated with the surrounding bone.
- Complications: Identifies potential complications such as graft resorption or infection.
- Treatment Planning: Informs treatment planning and helps optimize the timing of implant placement.
8.2 Assessing Tumor Growth In Cancer Treatment
Volume comparison is used to assess tumor growth and evaluate the effectiveness of cancer treatments. By comparing serial CT or MRI scans, clinicians can track changes in tumor size and shape.
- Process:
- Segmentation: Segment the tumor in each scan.
- Registration: Register the scans to correct for positioning errors.
- Volume Calculation: Calculate the volume of the tumor in each scan.
- Comparison: Compare the volumes to assess the tumor growth rate.
- Clinical Significance:
- Treatment Response: Indicates whether the tumor is responding to treatment.
- Progression: Identifies tumor progression and the need for alternative treatments.
- Survival: Can be used to predict patient survival.
8.3 Evaluating Lung Volume Changes In Respiratory Diseases
Volume comparison is used to evaluate lung volume changes in patients with respiratory diseases such as COPD and asthma. By comparing CT scans, clinicians can assess the severity of the disease and monitor the response to treatment.
- Process:
- Segmentation: Segment the lungs in each scan.
- Registration: Register the scans to correct for positioning errors.
- Volume Calculation: Calculate the volume of the lungs in each scan.
- Comparison: Compare the volumes to assess the lung volume changes.
- Clinical Significance:
- Disease Severity: Indicates the severity of the respiratory disease.
- Treatment Response: Monitors the response to treatment.
- Prognosis: Can be used to predict patient prognosis.
9. What Are The Future Trends In Volume Comparison Technology?
Future trends in volume comparison technology include artificial intelligence (AI), improved registration algorithms, and real-time volume analysis.
9.1 How Will Artificial Intelligence (AI) Enhance Volume Comparison?
AI has the potential to automate and improve many aspects of volume comparison, including segmentation, registration, and analysis.
- AI-Powered Segmentation:
- Automated Segmentation: AI algorithms can automatically segment ROIs with high accuracy and speed.
- Reduced Variability: AI can reduce inter- and intra-observer variability.
- Improved Efficiency: AI can significantly reduce the time required for segmentation.
- AI-Powered Registration:
- Improved Accuracy: AI algorithms can improve the accuracy of registration.
- Robustness: AI can make registration more robust to noise and artifacts.
- Adaptive Algorithms: AI can adapt to different types of images and anatomical structures.
- AI-Powered Analysis:
- Automated Analysis: AI algorithms can automatically analyze volume changes and identify clinically significant findings.
- Predictive Modeling: AI can be used to build predictive models for treatment outcomes.
9.2 Developments In Registration Algorithms For More Accurate Results
Ongoing developments in registration algorithms aim to improve accuracy, robustness, and efficiency.
- Deformable Registration:
- Advanced Algorithms: Advanced deformable registration algorithms can correct for complex deformations.
- Improved Accuracy: Improves the accuracy of volume comparisons in non-rigid structures.
- Multi-Modal Registration:
- Integration of Modalities: Integrates information from multiple imaging modalities (e.g., CT, MRI).
- Improved Accuracy: Improves the accuracy of registration and volume comparisons.
- Real-Time Registration:
- Real-Time Processing: Allows for real-time registration during scanning.
- Improved Efficiency: Improves efficiency and reduces the time required for analysis.
9.3 Real-Time Volume Analysis: What Is Its Potential?
Real-time volume analysis has the potential to transform clinical practice by providing immediate feedback during procedures.
- Applications:
- Surgical Planning: Provides real-time information during surgical planning.
- Image-Guided Surgery: Guides surgical procedures in real-time.
- Radiation Therapy: Monitors tumor response during radiation therapy.
- Benefits:
- Improved Accuracy: Improves the accuracy of surgical procedures and treatments.
- Reduced Complications: Reduces the risk of complications.
- Personalized Treatment: Allows for personalized treatment based on real-time feedback.
10. Frequently Asked Questions (FAQ) About Comparing Volumes
10.1 What Is The Best Software For Comparing Volumes?
3D Slicer is a popular choice due to its open-source nature and comprehensive features. Other options include Autodesk Meshmixer and CloudCompare, depending on specific needs.
10.2 How Can I Improve The Accuracy Of Volume Comparison?
Improve accuracy by standardizing scanning protocols, validating segmentation and registration results, and documenting the entire process.
10.3 What Are The Common Sources Of Error In Volume Comparison?
Common sources of error include segmentation errors, registration errors, image artifacts, and volume calculation errors.
10.4 How Does Registration Help In Volume Comparison?
Registration aligns pre- and post-operative scans, correcting for positioning discrepancies and ensuring accurate comparison of volumes.
10.5 What Is The Role Of Voxel Values In Measuring Volume Change?
Voxel values represent tissue density and are used to measure changes in relative density within the region of interest.
10.6 Can AI Be Used To Improve Volume Comparison?
Yes, AI can automate and improve segmentation, registration, and analysis, enhancing the accuracy and efficiency of volume comparison.
10.7 What Is The Significance Of Normalizing Voxel Values?
Normalizing voxel values reduces the effects of variations in scanner settings and patient-specific factors, improving the accuracy of comparison.
10.8 How Do I Choose The Right Statistical Test For Comparing Voxel Value Changes?
Choose a statistical test appropriate for your data, such as a paired t-test, Wilcoxon signed-rank test, or Kolmogorov-Smirnov test.
10.9 What Are The Future Trends In Volume Comparison Technology?
Future trends include AI-enhanced volume comparison, improved registration algorithms, and real-time volume analysis.
10.10 How Can I Monitor Bone Graft Healing Using Volume Comparison?
Monitor bone graft healing by segmenting the grafted area, registering pre- and post-operative scans, and calculating the volume change to assess bone growth.
Accurate volume comparison is crucial in various fields, from medical imaging to material science. By understanding the fundamental concepts, using the right tools, and applying appropriate techniques, you can achieve reliable and meaningful results. At COMPARE.EDU.VN, we provide comprehensive guides and resources to help you make informed decisions and comparisons.
Ready to make informed decisions? Visit compare.edu.vn today to explore detailed comparisons and reviews that will help you choose the best options for your needs. Whether you’re comparing products, services, or ideas, we’re here to help you make the right choice. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States or Whatsapp: +1 (626) 555-9090.
