Comparing the size of ions involves understanding the interplay between nuclear charge, electron shielding, and electron configuration. This analysis reveals how COMPARE.EDU.VN can aid in deciphering ionic properties. Through detailed comparisons, we provide clarity on predicting ionic dimensions and understanding isoelectronic series, enhancing your comprehension of these fundamental chemical concepts.
1. Understanding Ionic Size: Key Factors
Ionic size isn’t straightforward; it’s influenced by several factors. Let’s explore these factors and how they affect ionic radii.
1.1. What Factors Affect Ionic Size?
Several factors influence the size of ions, including nuclear charge, the number of electrons, and electron shielding. An ion’s size is determined by the balance between the attraction of the nucleus for its electrons and the repulsion between those electrons.
- Nuclear Charge: A higher nuclear charge pulls electrons closer, resulting in a smaller ion.
- Number of Electrons: More electrons increase electron-electron repulsion, expanding the ion’s size.
- Electron Shielding: Inner electrons shield outer electrons from the full nuclear charge, reducing the effective nuclear charge and increasing ionic size.
1.2. How Does Nuclear Charge Influence Ionic Size?
The nuclear charge, determined by the number of protons in the nucleus, exerts a strong influence on ionic size. As the nuclear charge increases, it pulls the electron cloud inward, thereby reducing the ionic radius.
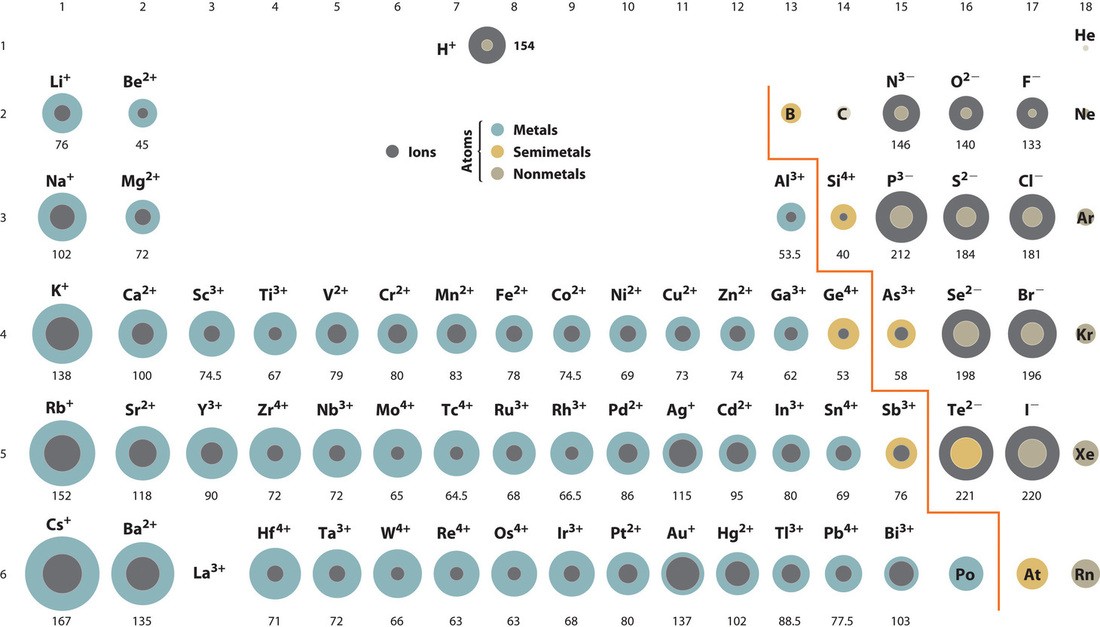
For example, consider the isoelectronic series (ions with the same number of electrons) such as ( N^{3-} ), ( O^{2-} ), ( F^{-} ), ( Na^{+} ), ( Mg^{2+} ), and ( Al^{3+} ). All these ions have 10 electrons, but their nuclear charges vary from +7 for nitrogen to +13 for aluminum. As the nuclear charge increases, the ionic radius decreases.
1.3. How Does Electron Configuration Affect Ionic Size?
Electron configuration plays a crucial role in determining the size of ions. The addition or removal of electrons alters the balance between electron-electron repulsion and nuclear attraction, thereby affecting the ionic radius.
When an atom loses electrons to form a cation, the electron-electron repulsion decreases, and the effective nuclear charge increases. Consequently, cations are smaller than their parent atoms. Conversely, when an atom gains electrons to form an anion, the electron-electron repulsion increases, and the effective nuclear charge decreases. This results in anions being larger than their parent atoms.
2. Trends in Ionic Size: The Periodic Table
Understanding the periodic trends in ionic size requires a look at how ionic radii change across periods and down groups in the periodic table.
2.1. What Are the Periodic Trends in Ionic Size?
Ionic size generally decreases across a period and increases down a group. These trends are similar to atomic radii trends but are influenced by the charge of the ions formed.
Across a period, the nuclear charge increases, pulling the electron cloud closer and reducing the ionic radius. Down a group, the principal quantum number ( n ) increases, meaning the outermost electrons are in higher energy levels and farther from the nucleus, leading to larger ionic radii.
2.2. How Does Ionic Size Change Across a Period?
Across a period, the ionic radius typically decreases due to the increasing nuclear charge. This trend is most evident in isoelectronic series, where ions have the same number of electrons but different numbers of protons.
For example, in the third period, consider the ions ( Na^{+} ), ( Mg^{2+} ), and ( Al^{3+} ). These ions are formed after the respective atoms lose electrons to achieve a noble gas configuration. The nuclear charge increases from +11 in sodium to +13 in aluminum, resulting in a significant decrease in ionic radii.
2.3. How Does Ionic Size Change Down a Group?
Down a group, the ionic radius increases due to the addition of electron shells. As the principal quantum number ( n ) increases, the valence electrons are located farther from the nucleus, leading to a larger ionic radius.
Consider the alkali metals (Group 1) as an example: ( Li^{+} ), ( Na^{+} ), ( K^{+} ), ( Rb^{+} ), and ( Cs^{+} ). The ionic radius increases from lithium to cesium as each subsequent element adds an additional electron shell, placing the valence electrons at greater distances from the nucleus.
3. Comparing Cations and Anions
Cations and anions exhibit distinct differences in size due to the gain or loss of electrons. Understanding these differences is crucial for predicting and explaining chemical properties.
3.1. Why Are Cations Smaller Than Their Parent Atoms?
Cations are always smaller than their parent atoms because the removal of electrons reduces electron-electron repulsion and increases the effective nuclear charge.
When an atom loses electrons to form a cation, the remaining electrons experience a stronger pull from the nucleus, leading to a contraction of the electron cloud. Additionally, the removal of electrons may also result in the loss of the outermost electron shell, further reducing the size of the ion.
3.2. Why Are Anions Larger Than Their Parent Atoms?
Anions are always larger than their parent atoms because the addition of electrons increases electron-electron repulsion and decreases the effective nuclear charge.
When an atom gains electrons to form an anion, the increased repulsion between electrons causes the electron cloud to expand. Moreover, the additional electrons increase the shielding effect, reducing the effective nuclear charge experienced by the valence electrons, which results in an increased ionic radius.
3.3. How Do You Compare the Sizes of Isoelectronic Ions?
Comparing the sizes of isoelectronic ions involves evaluating their nuclear charges. Isoelectronic ions have the same number of electrons, so the ion with the higher nuclear charge will be smaller because of the stronger pull on the electron cloud.
For instance, consider the isoelectronic series ( S^{2-} ), ( Cl^{-} ), ( K^{+} ), and ( Ca^{2+} ), all having 18 electrons. The nuclear charges are +16, +17, +19, and +20, respectively. Consequently, the ionic radii decrease in the order ( S^{2-} > Cl^{-} > K^{+} > Ca^{2+} ) due to the increasing nuclear charge.
4. Practical Examples of Ionic Size Comparison
Real-world examples illustrate how to apply the principles of ionic size comparison to predict and explain chemical phenomena.
4.1. Comparing the Sizes of Na+ and Mg2+
Sodium ion (( Na^{+} )) and magnesium ion (( Mg^{2+} )) are both commonly found in biological systems and chemical compounds. Comparing their sizes requires understanding their electron configurations and nuclear charges.
( Na^{+} ) has 10 electrons and a nuclear charge of +11, while ( Mg^{2+} ) also has 10 electrons but a nuclear charge of +12. Because they are isoelectronic, the higher nuclear charge of magnesium pulls the electrons closer, resulting in a smaller ionic radius compared to sodium. Thus, ( Mg^{2+} ) is smaller than ( Na^{+} ).
4.2. Comparing the Sizes of Cl- and K+
Chloride ion (( Cl^{-} )) and potassium ion (( K^{+} )) are important electrolytes in biological systems. Comparing their sizes requires considering their electron configurations and nuclear charges.
( Cl^{-} ) has 18 electrons and a nuclear charge of +17, while ( K^{+} ) also has 18 electrons but a nuclear charge of +19. Being isoelectronic, the greater nuclear charge of potassium draws the electrons more tightly, resulting in a smaller ionic radius compared to chloride. Therefore, ( K^{+} ) is smaller than ( Cl^{-} ).
4.3. How Does Ionic Size Affect Lattice Energy?
Ionic size significantly impacts lattice energy, which is the energy required to separate one mole of an ionic compound into its gaseous ions. Smaller ions with higher charges lead to greater lattice energies.
For example, consider sodium chloride (( NaCl )) and magnesium oxide (( MgO )). The ionic radii of ( Na^{+} ) and ( Cl^{-} ) are larger than those of ( Mg^{2+} ) and ( O^{2-} ), and magnesium and oxygen have higher charges. As a result, magnesium oxide has a much higher lattice energy than sodium chloride, making it a more stable compound.
5. Tools for Comparing Ionic Size on COMPARE.EDU.VN
COMPARE.EDU.VN provides tools and resources to assist in comparing ionic sizes, offering valuable insights for students, educators, and professionals.
5.1. How to Use the Ionic Size Comparison Tool
COMPARE.EDU.VN’s ionic size comparison tool allows users to input different ions and receive a detailed comparison of their sizes based on various factors such as nuclear charge, electron configuration, and position in the periodic table.
To use the tool, simply enter the ions you wish to compare. The tool will then provide a comprehensive analysis, including the ionic radii, electron configurations, and a visual representation of the ionic sizes relative to each other.
5.2. Accessing Data Tables of Ionic Radii
COMPARE.EDU.VN offers extensive data tables of ionic radii for various elements in different oxidation states. These tables are sourced from reliable scientific literature and are regularly updated to ensure accuracy.
Users can access these data tables to quickly find and compare the ionic radii of different elements. The data is presented in a clear and organized manner, making it easy to identify trends and make informed comparisons.
5.3. Educational Resources on Ionic Size
COMPARE.EDU.VN provides a range of educational resources, including articles, tutorials, and interactive simulations, to help users deepen their understanding of ionic size.
These resources cover topics such as the factors affecting ionic size, periodic trends in ionic radii, and the applications of ionic size in chemistry and materials science. Whether you are a student learning about ionic size for the first time or a professional seeking to refresh your knowledge, COMPARE.EDU.VN has something to offer.
6. Advanced Concepts in Ionic Size
Delving into advanced concepts provides a deeper understanding of ionic size and its implications in various fields.
6.1. Polarizing Power and Polarizability
Polarizing power refers to the ability of a cation to distort the electron cloud of an anion, while polarizability refers to the ease with which an anion’s electron cloud can be distorted. These concepts are closely related to ionic size.
Small, highly charged cations have high polarizing power because they exert a strong attraction on the electron cloud of nearby anions. Conversely, large, highly charged anions are easily polarized because their electron clouds are more diffuse and less tightly held by the nucleus.
6.2. Effects of Ionic Size on Crystal Structures
Ionic size plays a crucial role in determining the crystal structures of ionic compounds. The arrangement of ions in a crystal lattice is influenced by the relative sizes of the cations and anions.
For example, the rock salt structure (e.g., ( NaCl )) is favored when the cation and anion are of comparable size, while other structures, such as the cesium chloride structure (e.g., ( CsCl )), are favored when the cation is significantly larger than the anion.
6.3. Applications in Geochemistry and Mineralogy
Ionic size is an important factor in geochemistry and mineralogy, influencing the distribution of elements in the Earth’s crust and the formation of minerals.
The Goldschmidt rules, for example, describe how the size and charge of ions determine their compatibility in different mineral structures. Ions of similar size and charge can readily substitute for each other in minerals, while those with significantly different sizes or charges cannot.
7. Common Misconceptions About Ionic Size
Addressing common misconceptions about ionic size helps to clarify understanding and prevent errors in predictions and explanations.
7.1. Is Ionic Size Always Directly Related to Atomic Mass?
Ionic size is not always directly related to atomic mass. While there is a general trend of increasing size with increasing atomic mass down a group, other factors such as nuclear charge and electron configuration can have a greater influence.
For example, consider the isoelectronic series ( N^{3-} ), ( O^{2-} ), and ( F^{-} ). Nitrogen has a lower atomic mass than oxygen and fluorine, but ( N^{3-} ) is the largest ion due to its lower nuclear charge.
7.2. Can You Predict Ionic Size Solely Based on the Number of Electrons?
You cannot predict ionic size solely based on the number of electrons. While the number of electrons does influence electron-electron repulsion, the nuclear charge has a greater impact on the overall size of the ion.
For example, consider the ions ( Na^{+} ) and ( F^{-} ). Both ions have 10 electrons, but ( Na^{+} ) is significantly smaller than ( F^{-} ) due to its higher nuclear charge (+11 compared to +9 for fluorine).
7.3. Do All Ions of the Same Element Have the Same Size?
All ions of the same element do not have the same size. The size of an ion depends on its charge, which is determined by the number of electrons gained or lost.
For example, iron can form both ( Fe^{2+} ) and ( Fe^{3+} ) ions. The ( Fe^{3+} ) ion is smaller than the ( Fe^{2+} ) ion because it has a higher positive charge and experiences a greater effective nuclear charge.
8. Practical Applications of Understanding Ionic Size
The knowledge of ionic size has numerous practical applications across various fields, contributing to advancements in technology, medicine, and environmental science.
8.1. Applications in Battery Technology
Ionic size plays a critical role in battery technology, particularly in lithium-ion batteries. The size of the lithium ion (( Li^{+} )) affects its mobility through the electrolyte and its ability to intercalate into the electrode materials.
Smaller lithium ions can move more easily through the electrolyte and can be accommodated in a wider range of electrode materials, leading to improved battery performance. Researchers are continuously exploring new materials with optimal structures and ionic sizes to enhance battery capacity, charging rates, and overall efficiency.
8.2. Importance in Drug Delivery Systems
Ionic size is also important in drug delivery systems, where ions are used to encapsulate and transport drugs to specific locations within the body.
The size and charge of the ions used in these systems can affect their ability to cross cell membranes, their interactions with biological molecules, and their overall effectiveness in delivering the drug to the target site. By carefully selecting ions with appropriate sizes and charges, researchers can design drug delivery systems that are more effective and less toxic.
8.3. Role in Environmental Remediation
Ionic size plays a role in environmental remediation, where ions are used to remove pollutants from contaminated water and soil.
For example, certain types of clay minerals can selectively adsorb ions of specific sizes and charges, allowing them to be used to remove heavy metals and other contaminants from the environment. Understanding the interactions between ions and these materials is essential for developing effective remediation strategies.
9. Case Studies on Ionic Size
Examining case studies can provide deeper insights into how ionic size influences real-world phenomena and technological applications.
9.1. Case Study: Perovskite Solar Cells
Perovskite solar cells are a promising technology for renewable energy, and ionic size plays a critical role in their performance. The perovskite structure consists of a three-dimensional framework of ions, and the size of these ions affects the stability and efficiency of the solar cell.
Researchers have found that certain combinations of ions with appropriate sizes can lead to more stable and efficient perovskite structures. By carefully controlling the composition and structure of the perovskite material, it is possible to create high-performance solar cells that are both cost-effective and environmentally friendly.
9.2. Case Study: Zeolite Catalysts
Zeolite catalysts are widely used in the chemical industry, and ionic size is an important factor in their catalytic activity. Zeolites are crystalline materials with a porous structure, and the size of the pores can be controlled by varying the composition of the material.
Ions of specific sizes can be selectively adsorbed into the pores of the zeolite, where they can catalyze chemical reactions. By carefully designing the pore size and ionic composition of the zeolite, it is possible to create highly selective and efficient catalysts for a wide range of chemical processes.
9.3. Case Study: High-Temperature Superconductors
High-temperature superconductors are materials that exhibit superconductivity at relatively high temperatures, and ionic size is thought to play a role in their unique properties.
These materials typically contain complex structures with multiple types of ions, and the interactions between these ions can influence the electronic properties of the material. Researchers are still working to fully understand the role of ionic size in high-temperature superconductivity, but it is clear that this factor is important for achieving high-performance superconducting materials.
10. Future Trends in Ionic Size Research
The field of ionic size research is constantly evolving, with new discoveries and applications emerging all the time.
10.1. Advances in Computational Modeling
Advances in computational modeling are enabling researchers to more accurately predict and understand the behavior of ions in complex systems.
These models can take into account factors such as electron configuration, nuclear charge, and interionic interactions, providing valuable insights into the properties of ionic materials. As computational power continues to increase, these models will become even more sophisticated and useful for guiding the design of new materials and technologies.
10.2. Development of New Experimental Techniques
The development of new experimental techniques is also driving progress in ionic size research.
Techniques such as X-ray diffraction, neutron diffraction, and atomic force microscopy can provide detailed information about the structure and properties of ionic materials, allowing researchers to validate computational models and discover new phenomena. As these techniques continue to improve, they will provide even greater insights into the behavior of ions in complex systems.
10.3. Focus on Sustainable Materials
There is a growing focus on the development of sustainable materials, and ionic size is playing a key role in this effort.
Researchers are exploring new materials that are both environmentally friendly and high-performing, and they are using their knowledge of ionic size to design materials with optimal properties. By carefully selecting ions with appropriate sizes and charges, it is possible to create sustainable materials for a wide range of applications, from energy storage to environmental remediation.
Conclusion:
Understanding how to compare the size of ions is fundamental in chemistry, influencing properties from lattice energy to the design of new materials. Utilize COMPARE.EDU.VN to explore detailed comparisons, access data tables, and enhance your grasp of ionic radii.
Ready to make more informed decisions? Visit COMPARE.EDU.VN today to explore detailed comparisons and make the best choice for your needs. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090. Website: COMPARE.EDU.VN. Explore ionic radius, cation size, and anion size, and leverage our resources to make your decision-making process simpler and more effective.
 Ionic Radii of Common Elements
Ionic Radii of Common Elements
Frequently Asked Questions About Comparing Ionic Sizes
1. What is an isoelectronic series, and how does it help in comparing ionic sizes?
An isoelectronic series is a group of ions that have the same number of electrons but different numbers of protons (different nuclear charges). Comparing ions within an isoelectronic series helps determine how increasing nuclear charge affects ionic size. As the nuclear charge increases, the ionic size decreases because the positively charged nucleus pulls the electron cloud closer, resulting in a smaller radius.
2. Why are cations always smaller than their neutral atoms?
Cations are smaller than their neutral atoms because forming a cation involves the removal of one or more electrons. This reduction in the number of electrons decreases electron-electron repulsion, allowing the remaining electrons to be pulled closer to the nucleus by its positive charge. Additionally, the removal of electrons can also lead to the loss of the outermost electron shell, further reducing the size.
3. Why are anions always larger than their neutral atoms?
Anions are larger than their neutral atoms because forming an anion involves the addition of one or more electrons. This increase in the number of electrons increases electron-electron repulsion, causing the electron cloud to expand. The increased electron population also increases the shielding effect, reducing the effective nuclear charge experienced by the outermost electrons, which results in an increased ionic radius.
4. How does the position of an element on the periodic table relate to the size of its ions?
The position of an element on the periodic table can help predict the size of its ions based on periodic trends. Ionic size generally decreases across a period from left to right due to increasing nuclear charge and increases down a group due to the addition of electron shells. However, these trends can be modified by the specific charges and electron configurations of the ions.
5. What role does electron shielding play in determining ionic size?
Electron shielding refers to the phenomenon where inner electrons reduce the effective nuclear charge experienced by outer electrons. Greater shielding reduces the attractive force between the nucleus and the outer electrons, allowing the electron cloud to expand. Ions with greater shielding tend to be larger because the outer electrons are not as strongly held by the nucleus.
6. How does ionic size affect the properties of ionic compounds?
Ionic size significantly affects the properties of ionic compounds, such as lattice energy, melting point, and solubility. Smaller ions with higher charges generally lead to higher lattice energies and melting points because they can pack more closely together, resulting in stronger electrostatic attractions. Ionic size also affects solubility, as similarly sized ions tend to form more stable and soluble compounds.
7. Can ionic size be directly measured experimentally?
No, ionic size cannot be directly measured experimentally in the same way that one might measure the size of a macroscopic object. However, ionic radii can be estimated using X-ray diffraction and other experimental techniques that measure interionic distances in crystalline compounds. These distances can then be partitioned to estimate the radii of individual ions, considering factors such as charge and electron configuration.
8. How can I use COMPARE.EDU.VN to compare the sizes of different ions?
compare.edu.vn provides a variety of resources to compare ionic sizes, including detailed data tables, comparison tools, and educational materials. You can use the comparison tool to input specific ions and view their relative sizes, electron configurations, and nuclear charges. The data tables offer a comprehensive list of ionic radii for different elements in various oxidation states.
9. What is polarizing power, and how does it relate to ionic size?
Polarizing power is the ability of a cation to distort the electron cloud of an anion. Small, highly charged cations have high polarizing power because they can strongly attract and distort the electron cloud of nearby anions. This distortion can influence the properties of ionic compounds, such as their color, solubility, and reactivity.
10. How does ionic size influence the structure and properties of zeolites?
Ionic size plays a crucial role in determining the structure and properties of zeolites, which are crystalline materials with porous structures. The size and charge of the ions that make up the zeolite framework affect the size and shape of the pores, which in turn determine the zeolite’s selectivity for adsorbing molecules and its catalytic activity. By carefully controlling the ionic composition and structure of zeolites, it is possible to create materials with tailored properties for a wide range of applications.
