Comparing melting points effectively involves understanding the underlying principles that govern these physical properties. At COMPARE.EDU.VN, we provide a comprehensive guide to help you understand and compare melting points, enabling informed decisions across various scientific and practical applications. By considering factors such as intermolecular forces, molecular structure, and polarity, you can gain valuable insights. Understand phase transitions, thermal stability, and material characterization to make comparisons easier.
1. Understanding Melting Points: The Basics
1.1. What is a Melting Point?
The melting point is the temperature at which a substance transitions from a solid to a liquid state. This phase transition occurs when the substance absorbs enough energy to overcome the intermolecular forces (IMFs) holding its molecules in a fixed lattice structure. The melting point is a characteristic property of crystalline solids and is often used to identify substances and assess their purity.
1.2. Key Factors Influencing Melting Points
Several factors influence the melting point of a substance, including:
- Intermolecular Forces (IMFs): The strength of IMFs, such as van der Waals forces, dipole-dipole interactions, and hydrogen bonding, plays a crucial role. Stronger IMFs require more energy to overcome, resulting in higher melting points.
- Molecular Structure: The shape and size of molecules affect how closely they can pack together in the solid state. Compact, symmetrical molecules tend to have higher melting points compared to bulky, irregular ones.
- Molecular Weight: Generally, larger molecules with higher molecular weights have higher melting points due to increased van der Waals forces.
- Polarity: Polar molecules, which exhibit dipole-dipole interactions and hydrogen bonding, typically have higher melting points than nonpolar molecules of similar size and weight.
- Impurities: The presence of impurities in a substance can disrupt its crystal lattice, lowering the melting point and broadening the melting point range.
2. Intermolecular Forces and Melting Points
2.1. Van der Waals Forces
Van der Waals forces are weak, short-range attractive forces that arise from temporary fluctuations in electron distribution within molecules. These forces include London dispersion forces, dipole-dipole interactions, and dipole-induced dipole interactions.
- London Dispersion Forces: Present in all molecules, London dispersion forces are caused by instantaneous dipoles that arise from the random movement of electrons. Larger molecules with more electrons exhibit stronger London dispersion forces, leading to higher melting points.
- Dipole-Dipole Interactions: These forces occur between polar molecules that have permanent dipoles. The positive end of one molecule is attracted to the negative end of another, resulting in stronger interactions and higher melting points compared to nonpolar molecules with only London dispersion forces.
- Dipole-Induced Dipole Interactions: These forces occur when a polar molecule induces a temporary dipole in a nonpolar molecule, leading to a weak attraction.
2.2. Hydrogen Bonding
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on another electronegative atom. Substances capable of hydrogen bonding typically have significantly higher melting points than those with only van der Waals forces.
2.3. Ionic Bonding
Ionic compounds, which consist of positively and negatively charged ions held together by strong electrostatic forces, generally have very high melting points. The strength of the ionic bonds requires a significant amount of energy to overcome, resulting in high thermal stability.
2.4. Metallic Bonding
Metals consist of a lattice of positive ions surrounded by a “sea” of delocalized electrons. The strong attraction between the positive ions and the delocalized electrons results in metallic bonds, which are typically strong and lead to high melting points for many metals.
3. The Role of Molecular Structure
3.1. Molecular Shape and Packing Efficiency
The shape of a molecule significantly affects how efficiently it can pack in the solid state. Symmetrical molecules, such as spherical or cubic structures, tend to pack more closely together, leading to stronger IMFs and higher melting points. Irregularly shaped molecules, on the other hand, may not pack as efficiently, resulting in weaker IMFs and lower melting points.
3.2. Isomers and Melting Points
Isomers are molecules with the same chemical formula but different structural arrangements. The different shapes of isomers can affect their melting points. For example, branched isomers tend to have lower melting points than their straight-chain counterparts due to reduced packing efficiency.
3.3. Crystal Structure
The crystal structure of a solid also influences its melting point. Different crystal structures (e.g., simple cubic, face-centered cubic, body-centered cubic) have varying packing efficiencies and arrangements of molecules or atoms, which can affect the strength of IMFs and, consequently, the melting point.
4. Polarity and Melting Points
4.1. Dipole Moments and Intermolecular Interactions
Polar molecules have a net dipole moment due to the unequal sharing of electrons between atoms. The presence of a dipole moment leads to dipole-dipole interactions, which are stronger than London dispersion forces and result in higher melting points.
4.2. Hydrogen Bonding and Polar Molecules
As mentioned earlier, hydrogen bonding is a particularly strong intermolecular force that occurs between polar molecules containing hydrogen bonded to highly electronegative atoms. Substances capable of hydrogen bonding, such as water and alcohols, have significantly higher melting points compared to nonpolar molecules of similar size and weight.
4.3. Nonpolar Molecules and Melting Points
Nonpolar molecules, which have symmetrical charge distributions and no net dipole moment, primarily exhibit London dispersion forces. The strength of these forces depends on the size and shape of the molecule. Larger, more polarizable nonpolar molecules tend to have higher melting points than smaller, less polarizable ones.
5. How to Compare Melting Points Effectively
5.1. Identify the Types of Intermolecular Forces
The first step in comparing melting points is to identify the types of intermolecular forces present in each substance. Consider whether the molecules are nonpolar, polar, or capable of hydrogen bonding. Ionic and metallic compounds should also be considered, as they typically have very high melting points.
5.2. Assess Molecular Structure and Packing Efficiency
Evaluate the shape and size of the molecules to determine how efficiently they can pack in the solid state. Symmetrical, compact molecules tend to have higher melting points than bulky, irregularly shaped ones.
5.3. Consider Molecular Weight
In general, larger molecules with higher molecular weights have higher melting points due to increased van der Waals forces. However, this trend may not hold true if other factors, such as polarity or hydrogen bonding, are significant.
5.4. Account for Polarity and Dipole Moments
Polar molecules with dipole moments exhibit dipole-dipole interactions, which are stronger than London dispersion forces and result in higher melting points. Substances capable of hydrogen bonding typically have significantly higher melting points than those with only van der Waals forces.
5.5. Compare Experimental Melting Point Data
Whenever possible, compare experimental melting point data from reliable sources. Melting point data can provide valuable insights into the relative strengths of intermolecular forces and the purity of substances.
6. Factors Affecting the Accuracy of Melting Point Measurements
6.1. Impurities
The presence of impurities in a substance can significantly affect its melting point. Impurities disrupt the crystal lattice, lowering the melting point and broadening the melting point range. This phenomenon is known as melting point depression and is often used to assess the purity of organic compounds.
6.2. Heating Rate
The rate at which a substance is heated can also affect the accuracy of melting point measurements. Rapid heating may result in inaccurate melting point determinations, while slow heating allows for more precise measurements.
6.3. Calibration of Melting Point Apparatus
The accuracy of melting point measurements depends on the proper calibration of the melting point apparatus. Regular calibration with known standards is essential to ensure reliable results.
6.4. Sample Preparation
Proper sample preparation is crucial for accurate melting point measurements. The sample should be finely ground and packed tightly into the melting point capillary to ensure good thermal contact.
7. Practical Applications of Melting Point Comparisons
7.1. Material Identification
Melting points are characteristic properties of crystalline solids and are often used to identify substances. By comparing the experimental melting point of an unknown substance with known values, it is possible to identify the substance.
7.2. Purity Determination
The melting point range can be used to assess the purity of a substance. Pure substances typically have sharp, narrow melting point ranges, while impure substances exhibit broader melting point ranges and lower melting points.
7.3. Pharmaceutical Applications
Melting points are important in the pharmaceutical industry for characterizing and identifying drug substances, assessing their purity, and ensuring their stability during formulation and storage.
7.4. Polymer Science
In polymer science, melting points are used to characterize the thermal behavior of polymers, determine their degree of crystallinity, and assess their suitability for various applications.
7.5. Chemical Research
Melting points are routinely used in chemical research for identifying and characterizing new compounds, assessing the success of chemical reactions, and determining the stability of reaction products.
8. Examples of Melting Point Comparisons
8.1. Comparing Alkanes
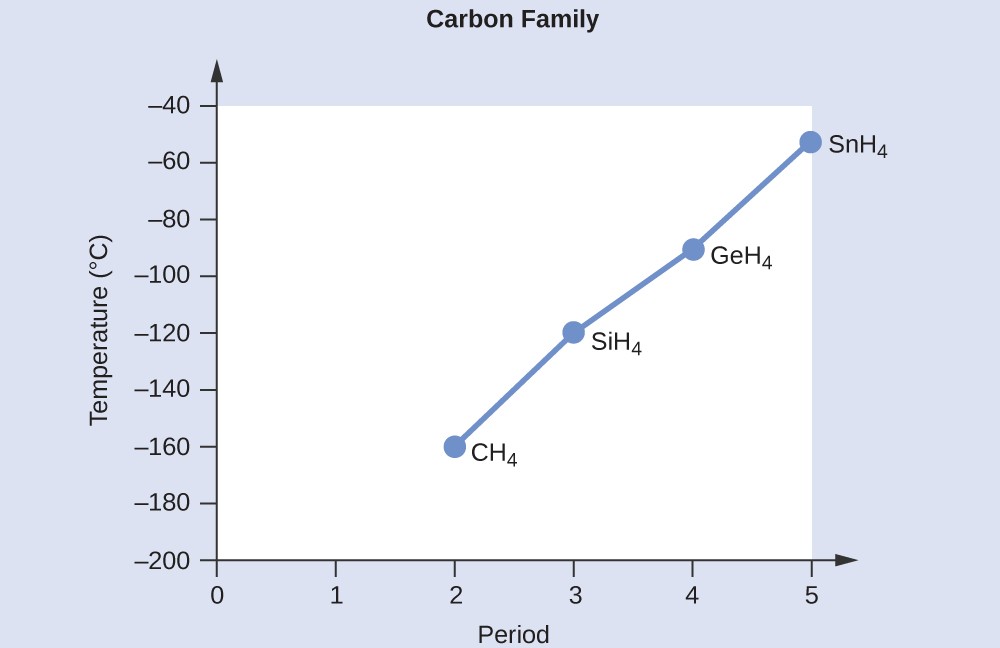
Alkanes are nonpolar hydrocarbons that exhibit only London dispersion forces. As the number of carbon atoms in an alkane increases, the molecular weight and surface area also increase, resulting in stronger London dispersion forces and higher melting points. For example, methane (CH4) has a melting point of -182.5 °C, while octane (C8H18) has a melting point of -56.8 °C.
8.2. Comparing Alcohols
Alcohols contain a hydroxyl (-OH) group, which allows for hydrogen bonding. As a result, alcohols have significantly higher melting points than alkanes of similar size and weight. For example, ethanol (CH3CH2OH) has a melting point of -114.1 °C, while ethane (CH3CH3) has a melting point of -183.2 °C.
8.3. Comparing Carboxylic Acids
Carboxylic acids contain a carboxyl (-COOH) group, which allows for both dipole-dipole interactions and hydrogen bonding. Carboxylic acids typically have higher melting points than alcohols and alkanes of similar size and weight. For example, acetic acid (CH3COOH) has a melting point of 16.6 °C.
8.4. Comparing Ionic Compounds
Ionic compounds, such as sodium chloride (NaCl) and magnesium oxide (MgO), have very high melting points due to the strong electrostatic forces between the ions. NaCl has a melting point of 801 °C, while MgO has a melting point of 2852 °C.
9. Common Mistakes to Avoid When Comparing Melting Points
9.1. Ignoring Intermolecular Forces
One of the most common mistakes is failing to consider the types and strengths of intermolecular forces present in each substance. Intermolecular forces play a critical role in determining melting points, and ignoring them can lead to inaccurate comparisons.
9.2. Overlooking Molecular Structure
Molecular structure, including shape and packing efficiency, can significantly affect melting points. Overlooking these factors can result in incorrect predictions.
9.3. Neglecting Polarity
Polarity and dipole moments influence intermolecular interactions and melting points. Neglecting polarity can lead to inaccurate comparisons, especially when comparing polar and nonpolar substances.
9.4. Relying Solely on Molecular Weight
While molecular weight can be a useful indicator, it should not be the only factor considered. Other factors, such as intermolecular forces and molecular structure, can be more significant in determining melting points.
9.5. Failing to Account for Impurities
Impurities can significantly lower melting points and broaden melting point ranges. Failing to account for impurities can lead to incorrect conclusions about the relative strengths of intermolecular forces.
10. Advanced Techniques for Melting Point Determination
10.1. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) is a thermal analysis technique that measures the heat flow associated with phase transitions, such as melting. DSC can provide accurate melting point determinations and information about the thermal behavior of substances.
10.2. X-Ray Diffraction (XRD)
X-ray diffraction (XRD) is a technique that provides information about the crystal structure of solids. XRD can be used to identify crystalline materials, determine their crystal structure, and assess their purity.
10.3. Thermal Microscopy
Thermal microscopy involves observing a sample under a microscope while controlling its temperature. This technique can be used to visualize phase transitions, such as melting, and to study the thermal behavior of materials.
11. The Influence of Pressure on Melting Points
11.1. Clapeyron Equation
The Clapeyron equation describes the relationship between pressure and melting point. In general, increasing pressure increases the melting point of most substances. However, for substances that contract upon melting (such as water), increasing pressure decreases the melting point.
11.2. High-Pressure Melting Point Measurements
High-pressure melting point measurements are used to study the behavior of materials under extreme conditions. These measurements can provide valuable insights into the properties of matter at high pressures and temperatures.
12. Predicting Melting Points: Empirical Rules and Computational Methods
12.1. Empirical Rules
Several empirical rules can be used to estimate melting points based on molecular structure and functional groups. These rules are often based on correlations between melting points and molecular properties, such as molecular weight, symmetry, and polarity.
12.2. Computational Methods
Computational methods, such as molecular dynamics simulations and density functional theory calculations, can be used to predict melting points based on the fundamental principles of physics and chemistry. These methods can provide accurate melting point predictions for a wide range of substances.
13. Melting Points in Nanomaterials
13.1. Size Effects
The melting points of nanomaterials, such as nanoparticles and nanowires, can be significantly different from those of bulk materials. Size effects, such as increased surface area and quantum confinement, can lower the melting points of nanomaterials.
13.2. Surface Energy
Surface energy plays a significant role in the melting behavior of nanomaterials. The high surface-to-volume ratio of nanomaterials results in increased surface energy, which can lower the melting point.
14. Polymorphism and Melting Points
14.1. Different Crystal Forms
Polymorphism refers to the ability of a substance to exist in multiple crystal forms, each with different physical properties, including melting point. Different polymorphs have different crystal structures and packing arrangements, which can affect their melting points.
14.2. Pharmaceutical Implications
Polymorphism is particularly important in the pharmaceutical industry, as different polymorphs of a drug substance can have different bioavailability, stability, and efficacy. Controlling the polymorphic form of a drug is essential for ensuring its quality and performance.
15. Eutectic Mixtures and Melting Points
15.1. Composition and Melting Behavior
A eutectic mixture is a mixture of two or more substances that has the lowest melting point compared to any other mixture composition. The eutectic point is the temperature at which the eutectic mixture melts.
15.2. Applications
Eutectic mixtures have various applications, including in soldering alloys, metal casting, and pharmaceutical formulations.
16. Applications of Melting Point Data in Forensics
16.1. Substance Identification
Melting point analysis is a valuable tool in forensic science for identifying unknown substances found at crime scenes. By comparing the melting point of an unknown substance with known values, forensic scientists can narrow down the possibilities and potentially identify the substance.
16.2. Drug Analysis
Melting point analysis is also used in drug analysis to identify and characterize illicit drugs. The melting point of a drug can provide important information about its identity and purity.
17. Recent Advances in Melting Point Research
17.1. New Materials
Ongoing research continues to explore the melting points of new materials, including novel polymers, composites, and nanomaterials. Understanding the melting behavior of these materials is essential for developing new technologies and applications.
17.2. Computational Modeling
Advances in computational modeling have led to more accurate and reliable methods for predicting melting points. These methods are increasingly used in materials science and engineering to design new materials with desired properties.
18. Case Studies: Real-World Examples of Melting Point Comparisons
18.1. Polymer Blends
Polymer blends are mixtures of two or more polymers. The melting behavior of polymer blends is complex and depends on the compatibility of the polymers, their molecular weights, and their crystal structures. Melting point comparisons are used to characterize the thermal behavior of polymer blends and to optimize their properties for various applications.
18.2. Metal Alloys
Metal alloys are mixtures of two or more metals. The melting points of metal alloys are often different from those of the pure metals. Melting point comparisons are used to design metal alloys with desired properties, such as high strength, corrosion resistance, and low melting point.
19. Conclusion: Mastering the Art of Melting Point Comparisons
Effectively comparing melting points involves a comprehensive understanding of the factors that influence these physical properties. By considering intermolecular forces, molecular structure, polarity, and experimental data, you can make informed decisions across various scientific and practical applications. Whether you’re identifying substances, assessing purity, or designing new materials, mastering the art of melting point comparisons will empower you to unlock valuable insights.
Ready to dive deeper and make more informed comparisons? Visit COMPARE.EDU.VN today for a wealth of resources and expert analysis to guide your decision-making process. Our platform offers detailed comparisons and objective assessments to help you choose the best options tailored to your specific needs.
Need personalized advice or have questions?
Contact us:
- Address: 333 Comparison Plaza, Choice City, CA 90210, United States
- WhatsApp: +1 (626) 555-9090
- Website: compare.edu.vn
20. FAQ: Frequently Asked Questions About Melting Points
20.1. What is the difference between melting point and freezing point?
The melting point is the temperature at which a substance transitions from solid to liquid, while the freezing point is the temperature at which a substance transitions from liquid to solid. For pure crystalline substances, the melting point and freezing point are the same.
20.2. How does molecular weight affect melting point?
Generally, larger molecules with higher molecular weights have higher melting points due to increased van der Waals forces. However, other factors, such as polarity and hydrogen bonding, can also play a significant role.
20.3. Why do impurities lower the melting point of a substance?
Impurities disrupt the crystal lattice of a substance, making it easier to overcome the intermolecular forces and transition to the liquid state. This results in a lower melting point and a broader melting point range.
20.4. What are intermolecular forces (IMFs)?
Intermolecular forces are attractive or repulsive forces that exist between molecules. These forces include van der Waals forces (London dispersion forces, dipole-dipole interactions, and dipole-induced dipole interactions) and hydrogen bonding.
20.5. How does hydrogen bonding affect melting point?
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on another electronegative atom. Substances capable of hydrogen bonding typically have significantly higher melting points than those with only van der Waals forces.
20.6. What is polymorphism, and how does it affect melting point?
Polymorphism refers to the ability of a substance to exist in multiple crystal forms, each with different physical properties, including melting point. Different polymorphs have different crystal structures and packing arrangements, which can affect their melting points.
20.7. What is a eutectic mixture, and how does it relate to melting points?
A eutectic mixture is a mixture of two or more substances that has the lowest melting point compared to any other mixture composition. The eutectic point is the temperature at which the eutectic mixture melts.
20.8. How is melting point used in material identification?
Melting points are characteristic properties of crystalline solids and are often used to identify substances. By comparing the experimental melting point of an unknown substance with known values, it is possible to identify the substance.
20.9. What is differential scanning calorimetry (DSC), and how is it used in melting point determination?
Differential scanning calorimetry (DSC) is a thermal analysis technique that measures the heat flow associated with phase transitions, such as melting. DSC can provide accurate melting point determinations and information about the thermal behavior of substances.
20.10. How does pressure affect the melting point of a substance?
In general, increasing pressure increases the melting point of most substances. However, for substances that contract upon melting (such as water), increasing pressure decreases the melting point.