Comparing the efficacy of two drugs can be complex, but COMPARE.EDU.VN simplifies the process by providing detailed comparisons and analyses. We offer an objective evaluation, utilizing established methodologies like adjusted indirect comparisons and mixed treatment comparisons, to help you understand the relative effectiveness of different medications. For a detailed understanding of treatment options, efficacy analysis, and medication comparison, explore COMPARE.EDU.VN today.
Table of Contents:
- Why Is Comparing Drug Efficacy Important?
- What Are Head-to-Head Clinical Trials?
- What is Naive Direct Comparison?
- What is Adjusted Indirect Comparison?
- What is Multiple Adjusted Indirect Comparisons?
- What is Mixed Treatment Comparisons?
- What Are Bayesian Statistical Models?
- What is the Role of Drug Regulatory Bodies?
- What are the Key Considerations When Comparing Drug Efficacy?
- How Can COMPARE.EDU.VN Help?
- FAQs About Comparing Drug Efficacy
1. Why Is Comparing Drug Efficacy Important?
Comparing the effectiveness of two drugs is essential for making informed decisions about healthcare treatments. Evaluating therapeutic effectiveness, treatment options, and medication comparison plays a crucial role in clinical practice, public health, and health policy.
- Clinical Practice: When multiple drug options are available, understanding their relative efficacies helps clinicians choose the most appropriate treatment for their patients. This ensures that patients receive the most effective medication, improving their health outcomes.
- Public Health: Public health organizations need to make decisions about which drugs to include in formularies and treatment guidelines. Comparative efficacy data informs these decisions, ensuring that resources are allocated to the most effective treatments, thereby improving the overall health of the population.
- Health Policy: Health policy makers rely on comparative efficacy data to develop policies related to drug pricing, reimbursement, and access. Understanding the relative benefits of different drugs helps policymakers make informed decisions that promote efficient use of healthcare resources.
Without a clear understanding of relative efficacies, healthcare decisions may be suboptimal, leading to poorer patient outcomes and inefficient resource allocation. For example, in the treatment of type 2 diabetes mellitus (T2DM), the introduction of new drug classes like glucagon-like peptide-1 (GLP-1) analogues and dipeptidyl peptidase 4 (DPP4) inhibitors has complicated therapeutic choices. Knowing the relative efficacies of these agents is critical for determining their place in the overall treatment pathway.
2. What Are Head-to-Head Clinical Trials?
Head-to-head clinical trials directly compare two or more treatments in the same study to assess their relative efficacy and safety. In these trials, participants are randomly assigned to receive one of the treatments being compared, and outcomes are measured to determine which treatment performs better.
- Advantages:
- Direct Comparison: Provides a direct comparison of the treatments under the same experimental conditions, minimizing confounding factors.
- Strong Evidence: Offers strong evidence for decision-making in clinical practice and health policy.
- Disadvantages:
- Costly: Requires large sample sizes and significant resources, making them expensive to conduct.
- Time-Consuming: Takes considerable time to design, execute, and analyze.
- Limited Availability: Often lacking due to the expense and complexity involved in conducting them.
Due to the high cost and complexity, head-to-head trials are not always feasible, leading to gaps in knowledge about the relative efficacies of different treatments. In many cases, drug registration relies on placebo-controlled trials, which demonstrate efficacy compared to a placebo but do not provide direct comparisons between active treatments. Therefore, alternative methods for comparing drug efficacy are necessary when head-to-head trial data is lacking.
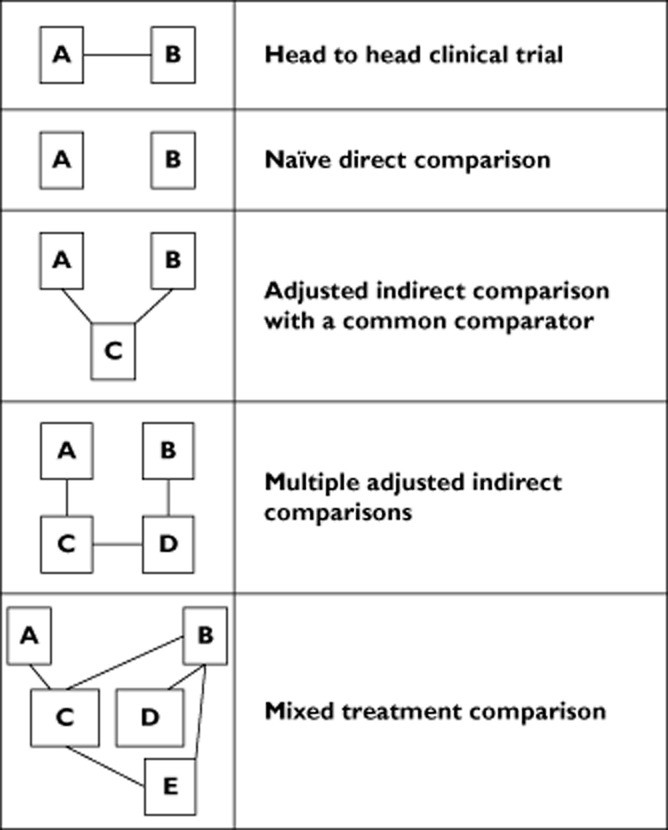
3. What is Naive Direct Comparison?
Naive direct comparison refers to directly comparing clinical trial results for one drug with those of another, without adjusting for differences in trial designs, populations, or comparators. This method involves a straightforward comparison of outcomes reported in different studies.
- How it Works: Outcomes from separate clinical trials of different drugs are directly compared without any statistical adjustments.
- Example: A meta-analysis directly comparing various oral anti-diabetic agents with respect to glycemic control, without adjusting for the control treatment’s level of glycemic control.
Conceptualization of the methods to compare the relative efficacies of drugs (or other interventions)
- Advantages:
- Simplicity: Easy to understand and implement, requiring no complex statistical adjustments.
- Exploratory Insights: Can provide initial insights and generate hypotheses for further investigation.
- Disadvantages:
- Bias Risk: High risk of bias due to differences in study populations, comparators, and outcome measurements.
- Confounding Factors: Susceptible to confounding, as differences in outcomes may be attributable to factors other than the drugs themselves.
- Inaccurate Results: May lead to inaccurate conclusions about the relative efficacy of different treatments.
The major limitation of naive direct comparison is the inability to determine whether observed differences are solely attributable to the drugs themselves. Differences may reflect variations in trial aspects such as populations, comparators, and outcomes. Conversely, similar efficacies may be inaccurately found due to variations masking true differences. As Bucher et al. claimed, naive direct comparisons break the original randomization and are subject to significant confounding and bias because of systematic differences among the trials being compared.
4. What is Adjusted Indirect Comparison?
Adjusted indirect comparison is a statistical method used to compare the effects of two treatments by comparing each to a common comparator. This approach preserves the randomization of originally assigned patient groups and estimates the treatment effect between two treatments relative to a shared reference.
- How it Works: Two drugs (A and B) are compared indirectly through a common comparator (C). Drug A is compared to Drug C in one trial, and Drug B is compared to Drug C in another. The difference between A and B is estimated by comparing the differences between A and C and between B and C.
- Example: Comparing two hypoglycemic drugs (A and B) for blood glucose reduction, using another drug (C) as the common comparator.
Table 1: Adjusted Indirect Comparison and Naive Direct Comparison (Continuous Outcomes)
| Clinical Trial 1 | Clinical Trial 2 | |
|---|---|---|
| A | C | |
| Observed Change | -3 mmol/L | -2 mmol/L |
| Adjusted Indirect Comp. | [(−3) − (−2)] − [(−2) − (−1)] = 0 mmol/L | |
| Naive Direct Comparison | (−3) − (−2) = -1 mmol/L |
Adjusted indirect comparison and naive direct comparison: hypothetical example using continuous outcomes. Two hypoglycaemic drugs, A and B, have been assessed with respect to reduction in blood glucose. Drug A was compared with drug C in a head-to-head clinical trial and drug B with drug C in another
- Advantages:
- Maintains Randomization: Preserves the randomization of patient groups, reducing bias.
- Uses Common Comparator: Employs a common comparator to link the two treatments, allowing for a fair comparison.
- Disadvantages:
- Uncertainty: Associated with uncertainty, as the statistical uncertainties of component comparison studies are summed.
- Assumption of Similarity: Relies on the assumption that subjects recruited into the different studies are similar enough to be pooled.
Adjusted indirect comparison is accepted by drug reimbursement agencies like the Australian Pharmaceutical Benefits Advisory Committee (PBAC), the UK National Institute of Clinical Excellence (NICE), and the Canadian Agency for Drugs and Technologies in Health (CADTH). However, this method’s main disadvantage is the uncertainty that arises from summing the statistical uncertainties of the component comparison studies.
Table 2: Adjusted Indirect Comparison and Naive Direct Comparison (Binary Outcomes)
| Clinical Trial 1 | Clinical Trial 2 | |
|---|---|---|
| A | C | |
| Patients Reaching Goal | 30% | 15% |
| Adjusted Indirect Comp. | (30%/15%) / (20%/10%) = 1.0 | |
| Naive Direct Comparison | 30%/20% = 1.5 |
Adjusted indirect comparison and naive direct comparison: hypothetical example using binary outcomes. Two hypoglycaemic drugs, A and B, have been assessed with respect to reduction in blood glucose. Drug A was compared with drug C in a head-to-head clinical trial and drug B with drug C in another
5. What is Multiple Adjusted Indirect Comparisons?
Multiple adjusted indirect comparisons extend the concept of adjusted indirect comparisons to situations where no single common comparator exists. Instead, a series of links is constructed, where the two drugs of interest are indirectly linked via two or more comparators.
- How it Works: Drug A is compared with drug C, and drug B with drug D. Drugs C and D are directly compared in another trial, allowing for an indirect comparison of A vs. B through their direct links to C and D.
- Example: Comparing sitagliptin with insulin in T2DM by linking them via sitagliptin vs. placebo, exenatide vs. placebo, and insulin vs. exenatide trials.
Table 3: Example of a Multiple Adjusted Indirect Comparison
| Sitagliptin vs. Placebo | Exenatide vs. Placebo | Insulin vs. Exenatide | |
|---|---|---|---|
| Results (Absolute Change in HbA1c) | 0.57 (95% CI: 0.45, 0.72) | 0.52 (95% CI: 0.32, 0.92) | 0.40 (95% CI: 0.27, 0.60) |
| Adjusted Indirect Comparison: Sitagliptin vs. Exenatide | 0.30 (95% CI: 0.17, 0.53) | ||
| Adjusted Indirect Comparison: Sitagliptin vs. Insulin | 0.74 (95% CI: 0.40, 1.37) |
Example of a multiple adjusted indirect comparison Kim et al. In order to compare sitagliptin with insulin in T2DM with respect to change in HbA1c, links were made via sitagliptin vs. placebo, exenatide vs. placebo and insulin vs. exenatide
- Advantages:
- Accommodates Complex Scenarios: Enables comparisons when no single common comparator is available.
- Utilizes Available Data: Maximizes the use of existing trial data to make indirect inferences.
- Disadvantages:
- Increased Uncertainty: Uncertainty accumulates at every link, potentially leading to significant overall uncertainty.
- Complexity: Requires careful selection of trials and linking comparators to minimize bias and confounding.
With multiple indirect comparisons, uncertainty accumulates at each link. The extent of uncertainty correlates with the number of links in the series. The key underlying assumption is that subjects recruited into the different studies being linked via the common comparator are similar enough to be pooled.
6. What is Mixed Treatment Comparisons?
Mixed treatment comparison (MTC) is a statistical method that combines direct and indirect evidence to compare multiple treatments simultaneously. It uses Bayesian models to incorporate all available data for a drug, even data not pertaining to the specific pairwise comparison of interest.
- How it Works: Any trial including either one of two drugs being compared provides information for the comparison between the two, regardless of the comparators. This method accommodates direct and indirect information about treatment effects.
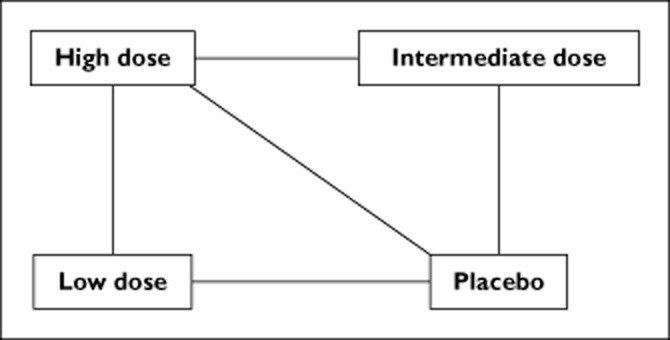
- Example: Investigating the impact of statin dose (high, intermediate, or low) on major cardiovascular events using evidence from 47 trials that compared different statin dose categories with either placebo or another dose category.
Conceptualization of a mixed treatment comparison undertaken by Ribeiro et al., who investigated the impact of various statin doses (high, intermediate or low) and placebo on major cardiovascular events using evidence from 47 trials. Lines indicate the intervention groups that had been directly compared in the trials
- Advantages:
- Comprehensive Analysis: Considers the totality of evidence, including both direct and indirect data.
- Reduced Uncertainty: Generally reduces uncertainty compared to adjusted indirect comparisons due to the incorporation of additional data.
- Disadvantages:
- Complexity: Requires advanced statistical methods and expertise in Bayesian models.
- Heterogeneity: Susceptible to non-statistical heterogeneity, necessitating subjective assessments of which studies to pool.
- Limited Acceptance: Not universally accepted by drug regulatory bodies, reflecting its relative novelty.
The major advantage of MTCs over adjusted indirect analyses is that they accommodate direct as well as indirect information about the treatment effects of drugs. As a result of additional data being incorporated and the underlying assumptions of Bayesian theory, uncertainty associated with MTCs is generally less than that of adjusted indirect comparisons.
Table 4: Results of Direct, Adjusted Indirect, and Mixed Treatment Comparisons
| High vs. Low Dose | High vs. Intermediate Dose | Intermediate vs. Low Dose | |
|---|---|---|---|
| Direct Comparison | 1.00 (0.56, 1.79) | 0.86 (0.77, 0.96) | Not Available |
| Adjusted Indirect Comparison | 0.87 (0.66, 1.14) | 1.02 (0.78, 1.35) | 0.85 (0.82, 0.87) |
| Mixed Treatment Comparison | 0.83 (0.68, 0.99) | 0.91 (0.80, 1.04) | 0.91 (0.76, 1.09) |
Results of a direct comparison, adjusted indirect comparison and mixed treatment comparison of various dose categories of statins and the relative risk of stroke: key results from Ribeiro et al.
7. What Are Bayesian Statistical Models?
Bayesian statistical models are a class of statistical models that use Bayesian inference to update the probability estimate for a hypothesis as more evidence is acquired. Unlike frequentist statistics, which treat probabilities as frequencies of events, Bayesian statistics treat probabilities as degrees of belief.
- Key Features:
- Prior Beliefs: Incorporate prior beliefs or knowledge about parameters through prior distributions.
- Updating with Data: Update these prior beliefs with observed data to obtain posterior distributions.
- Probability Distributions: Provide probability distributions for parameters, reflecting uncertainty.
- Applications in MTC: In mixed treatment comparisons, Bayesian models allow for the incorporation of both direct and indirect evidence to estimate treatment effects. They provide a framework for combining data from multiple sources and quantifying uncertainty.
Bayesian models are complex and require theoretical and technical know-how. Expertise in MTC is growing, and software like ‘WinBUGS’ (developed by the Medical Research Council Biostatistics Unit from Cambridge University) is available for undertaking MTCs.
8. What is the Role of Drug Regulatory Bodies?
Drug regulatory bodies play a critical role in assessing and approving new drugs, as well as monitoring their safety and efficacy post-market. These bodies establish guidelines and standards for clinical trials, data analysis, and drug labeling.
- Key Responsibilities:
- Evaluation of Clinical Trial Data: Reviewing data from clinical trials to assess the safety and efficacy of new drugs.
- Setting Standards: Establishing standards for drug manufacturing, quality control, and labeling.
- Post-Market Surveillance: Monitoring drugs for adverse events and ensuring continued safety and efficacy.
- Acceptance of Indirect Comparisons: Regulatory bodies vary in their acceptance of indirect comparison methods. Some, like the US Food and Drug Administration (FDA), specifically mention adjusted indirect comparisons in their guidelines, while others do not explicitly address mixed treatment comparisons (MTCs).
Drug reimbursement agencies, such as CADTH and NICE, may accept MTCs as part of drug reimbursement submissions, whereas others, like the PBAC, remain uncertain. The level of acceptance often depends on the maturity of the method and the perceived reliability of the results.
9. What are the Key Considerations When Comparing Drug Efficacy?
When comparing the efficacy of two drugs, several key considerations must be taken into account to ensure a fair and accurate assessment. These considerations include:
- Study Population: Ensuring that the populations in the different studies being compared are similar enough to be pooled. Differences in patient characteristics can affect treatment outcomes and introduce bias.
- Comparator Treatments: Accounting for differences in comparator treatments used in the trials. The choice of comparator can influence the observed treatment effect and affect the comparability of results.
- Outcome Measures: Ensuring that the outcome measures used in the trials are consistent and well-defined. Differences in outcome definitions or measurement methods can make it difficult to compare results across studies.
- Statistical Methods: Using appropriate statistical methods to adjust for differences in study designs and populations. Adjusted indirect comparisons and mixed treatment comparisons can help to minimize bias and improve the accuracy of the results.
- Heterogeneity: Assessing heterogeneity (variability) among the trials being compared. High heterogeneity can indicate that the trials are too different to be meaningfully combined, necessitating careful consideration of the reasons for the variability.
Failing to address these considerations can lead to inaccurate conclusions about the relative efficacy of different drugs. Careful attention to these factors is essential for making informed decisions about treatment choices.
10. How Can COMPARE.EDU.VN Help?
COMPARE.EDU.VN offers comprehensive resources and tools to help you compare the efficacy of different drugs. Our platform provides:
- Detailed Comparisons: Access thorough analyses and comparisons of various medications, highlighting their strengths and weaknesses.
- Objective Evaluations: Benefit from unbiased evaluations based on established methodologies, including adjusted indirect comparisons and mixed treatment comparisons.
- User-Friendly Interface: Navigate our user-friendly interface to easily find the information you need and make informed decisions.
- Up-to-Date Information: Stay informed with the latest research and clinical trial data, ensuring you have the most current information available.
Whether you are a healthcare professional, a patient, or a policymaker, COMPARE.EDU.VN empowers you to make informed decisions about treatment options.
Ready to make informed healthcare decisions? Visit COMPARE.EDU.VN today for detailed drug comparisons and objective evaluations. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090.
FAQs About Comparing Drug Efficacy
-
What is the best method for comparing drug efficacy?
The best method depends on the availability of data. Head-to-head trials are ideal, but when they are lacking, adjusted indirect comparisons or mixed treatment comparisons can be used. -
Are naive direct comparisons reliable?
No, naive direct comparisons are generally unreliable due to the high risk of bias and confounding factors. -
What is the key assumption in adjusted indirect comparisons?
The key assumption is that the subjects recruited into the different studies being linked via the common comparator are similar enough to be pooled. -
What is a mixed treatment comparison (MTC)?
MTC is a statistical method that combines direct and indirect evidence to compare multiple treatments simultaneously, using Bayesian models. -
Why is uncertainty a concern in indirect comparisons?
Uncertainty arises from summing the statistical uncertainties of the component comparison studies, potentially leading to less precise estimates of treatment effects. -
How do drug regulatory bodies view indirect comparisons?
Regulatory bodies vary in their acceptance; some, like the FDA, mention adjusted indirect comparisons, while others are more cautious about MTCs. -
What is the role of Bayesian models in MTC?
Bayesian models allow for the incorporation of both direct and indirect evidence, providing a framework for combining data from multiple sources and quantifying uncertainty. -
What factors should be considered when comparing drug efficacy?
Key factors include study population, comparator treatments, outcome measures, statistical methods, and heterogeneity among trials. -
How can COMPARE.EDU.VN help me compare drug efficacy?
COMPARE.EDU.VN offers detailed comparisons, objective evaluations, a user-friendly interface, and up-to-date information to help you make informed decisions. -
Where can I find reliable information about drug comparisons?
Visit compare.edu.vn for comprehensive and objective evaluations of different drugs.