Comparing boiling points of compounds involves understanding the intermolecular forces, molecular weight, and molecular symmetry that influence these physical properties, COMPARE.EDU.VN provides a detailed analysis of these factors to help you make informed comparisons. Delve into the intricacies of intermolecular attractions, explore trends in molecular size, and learn how molecular shape impacts boiling point using our comprehensive guide for identifying physical characteristics, chemical properties, and phase transitions.
1. Understanding Intermolecular Forces and Boiling Points
Intermolecular forces are crucial in determining the boiling points of compounds. The stronger these forces, the more energy is required to overcome them, leading to a higher boiling point. The primary intermolecular forces are:
- Ionic interactions
- Hydrogen bonding
- Dipole-dipole interactions
- Van der Waals dispersion forces
The relative strength of these forces typically follows the order: Ionic > Hydrogen bonding > Dipole-dipole > Van der Waals dispersion forces.
To effectively compare boiling points, consider the functional groups present in the molecules. For example, let’s compare a few butane alcohol derivatives. Diethyl ether (C4H10O) molecules are held together by dipole-dipole interactions due to the polarized C-O bonds. Its isomer, 1-butanol, has a significantly higher boiling point because it contains a hydroxyl group capable of hydrogen bonding. Sodium butoxide, a salt, has extremely strong ionic interactions, causing it to decompose before it can boil. Butane (C4H10), lacking polar functional groups, exhibits only weak Van der Waals dispersion forces, resulting in a very low boiling point.
Key Takeaway: For molecules with similar molecular weights, boiling points are largely determined by the types of functional groups present and the resulting intermolecular forces.
1.1. How do intermolecular forces influence boiling points?
Intermolecular forces (IMFs) are attractive or repulsive forces between molecules. They dictate how molecules interact with each other and, consequently, how much energy (in the form of heat) is needed to separate them from the liquid phase into the gaseous phase. Stronger IMFs result in higher boiling points because more energy is needed to overcome these attractions.
1.2. What are the different types of intermolecular forces?
There are several types of IMFs, each with varying strengths:
- Van der Waals Forces (London Dispersion Forces): These are the weakest IMFs, present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles. Larger molecules with more electrons exhibit stronger London dispersion forces.
- Dipole-Dipole Interactions: These forces occur between polar molecules, which have a permanent dipole moment due to uneven electron distribution. The positive end of one molecule attracts the negative end of another.
- Hydrogen Bonding: This is a particularly strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms such as nitrogen (N), oxygen (O), or fluorine (F). Hydrogen bonds are stronger than typical dipole-dipole interactions and significantly elevate boiling points.
- Ionic Interactions: These are the strongest IMFs, occurring between ions in ionic compounds. The strong electrostatic attraction between oppositely charged ions results in very high melting and boiling points.
1.3. How does hydrogen bonding affect boiling points?
Hydrogen bonding significantly elevates boiling points. Molecules capable of hydrogen bonding require considerably more energy to transition into the gaseous phase compared to molecules with only weaker IMFs. For instance, water (H2O) has a much higher boiling point than methane (CH4) because water molecules form extensive hydrogen bonds.
1.4. How do dipole-dipole interactions influence boiling points?
Dipole-dipole interactions increase boiling points relative to compounds with only London dispersion forces. The presence of a permanent dipole moment in a molecule leads to stronger attractions between molecules, requiring more energy to overcome during boiling.
1.5. How do Van der Waals forces impact boiling points?
Van der Waals forces, specifically London dispersion forces, are present in all molecules and contribute to boiling points. The strength of these forces increases with molecular size and surface area. Larger molecules have more electrons and a greater surface area for temporary dipoles to form, resulting in higher boiling points.
2. Molecular Weight and Boiling Point Trends
For molecules with a given functional group, boiling point generally increases with molecular weight. This trend is due to the increasing strength of Van der Waals dispersion forces as molecular size increases. These forces are proportional to the surface area of the molecule.
As the length of the carbon chain increases, the surface area also increases, enhancing the ability of individual molecules to attract each other. Think of long molecules as strands of spaghetti; the longer the strands, the more energy it takes to pull them apart. These longer chains have more regions where they can align closely, maximizing the effect of Van der Waals forces.
2.1. Why does boiling point increase with molecular weight?
As molecular weight increases, the number of electrons in a molecule also increases. This leads to greater polarizability, meaning the electron cloud is more easily distorted to form temporary dipoles. These temporary dipoles result in stronger London dispersion forces, which require more energy to overcome, thus increasing the boiling point.
2.2. How do alkanes demonstrate the relationship between molecular weight and boiling point?
Alkanes provide a clear example of how boiling point increases with molecular weight. As the number of carbon atoms in an alkane increases, so does its boiling point. For instance, methane (CH4) has a much lower boiling point than ethane (C2H6), which in turn has a lower boiling point than propane (C3H8), and so on.
2.3. What role does surface area play in this trend?
Surface area is a critical factor in the relationship between molecular weight and boiling point. Larger molecules have greater surface areas, allowing for more contact points between molecules. This increased contact enhances the effect of Van der Waals forces, making it more difficult to separate the molecules and thus raising the boiling point.
2.4. Are there exceptions to the rule that boiling point increases with molecular weight?
While the general trend is that boiling point increases with molecular weight, there are exceptions. Molecular shape and branching can significantly affect intermolecular forces. For example, isomers with more branching tend to have lower boiling points than their straight-chain counterparts, even though they have the same molecular weight.
2.5. How can this trend be used to predict the boiling points of unknown compounds?
By understanding the relationship between molecular weight and boiling point, you can estimate the boiling points of unknown compounds, especially within homologous series like alkanes or alcohols. This estimation is most accurate when comparing compounds with similar structures and functional groups.
3. The Impact of Molecular Symmetry and Branching on Boiling Points
Molecular symmetry and branching play a crucial role in determining boiling points. Highly symmetrical molecules often have lower boiling points compared to their less symmetrical counterparts. This is because symmetrical molecules have a smaller surface area available for intermolecular interactions. Branching reduces the surface area of a molecule, leading to weaker Van der Waals forces and lower boiling points.
Consider the isomers pentane (36°C) and 2,2-dimethylpropane (9°C). Pentane, with its straight chain, has a larger surface area for intermolecular interactions, resulting in a higher boiling point than the more spherical 2,2-dimethylpropane.
This effect also applies to molecules capable of hydrogen bonding, such as alcohols. The hydroxyl group in 1-pentanol is more exposed than in 3-pentanol, allowing for more effective hydrogen bonding and a higher boiling point.
3.1. How does molecular shape influence boiling points?
Molecular shape significantly impacts boiling points by affecting the surface area available for intermolecular interactions. Linear molecules have larger surface areas, allowing for greater contact and stronger Van der Waals forces, leading to higher boiling points. Spherical or highly branched molecules have smaller surface areas and reduced intermolecular contact, resulting in lower boiling points.
3.2. What is the effect of branching on boiling points?
Branching reduces the surface area of a molecule, which in turn weakens the Van der Waals forces. Branched isomers have lower boiling points compared to their straight-chain counterparts with the same molecular weight because the reduced surface area limits the extent of intermolecular interactions.
3.3. Why do symmetrical molecules often have lower boiling points?
Symmetrical molecules tend to pack more efficiently in the solid state, leading to higher melting points, but they have less surface area available for intermolecular interactions in the liquid state, resulting in lower boiling points. The symmetry reduces the overall intermolecular forces that need to be overcome during boiling.
3.4. Can you provide examples of isomers that illustrate this concept?
Isomers like n-pentane and neopentane (2,2-dimethylpropane) clearly illustrate this concept. N-pentane is a straight-chain alkane with a relatively high boiling point because of its larger surface area. Neopentane, a highly branched isomer, has a much lower boiling point due to its spherical shape and reduced surface area.
3.5. How does the position of functional groups affect boiling points?
The position of functional groups, especially in alcohols, can influence boiling points. When a functional group is more exposed (e.g., at the end of a carbon chain), it can participate more effectively in intermolecular interactions like hydrogen bonding. Conversely, functional groups that are sterically hindered (e.g., located in the middle of a branched chain) are less able to form strong intermolecular bonds, leading to lower boiling points.
4. Comparative Analysis of Functional Groups and Boiling Points
Different functional groups exhibit different types and strengths of intermolecular forces, which directly affect boiling points. Here’s a comparative analysis:
| Functional Group | Intermolecular Forces | Boiling Point Trend |
|---|---|---|
| Alkanes | Van der Waals dispersion forces | Low; increases with molecular weight |
| Alkenes/Alkynes | Van der Waals dispersion forces | Similar to alkanes with comparable molecular weight |
| Ethers | Dipole-dipole, Van der Waals dispersion forces | Higher than alkanes; lower than alcohols |
| Aldehydes/Ketones | Dipole-dipole, Van der Waals dispersion forces | Higher than ethers; lower than alcohols |
| Alcohols | Hydrogen bonding, Dipole-dipole, Van der Waals forces | High; significantly higher than ethers and aldehydes/ketones |
| Carboxylic Acids | Hydrogen bonding (dimers), Dipole-dipole, Van der Waals forces | Very high; often higher than alcohols due to dimer formation |
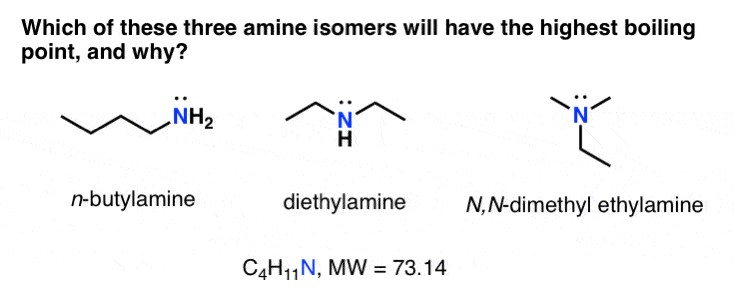
| Amines | Hydrogen bonding, Dipole-dipole, Van der Waals forces | High; lower than alcohols due to weaker hydrogen bonding in amines |
| Amides | Hydrogen bonding, Dipole-dipole, Van der Waals forces | Very high; capable of strong hydrogen bonding |
| Halides | Dipole-dipole, Van der Waals dispersion forces | Higher than alkanes; increases with the size of the halogen |
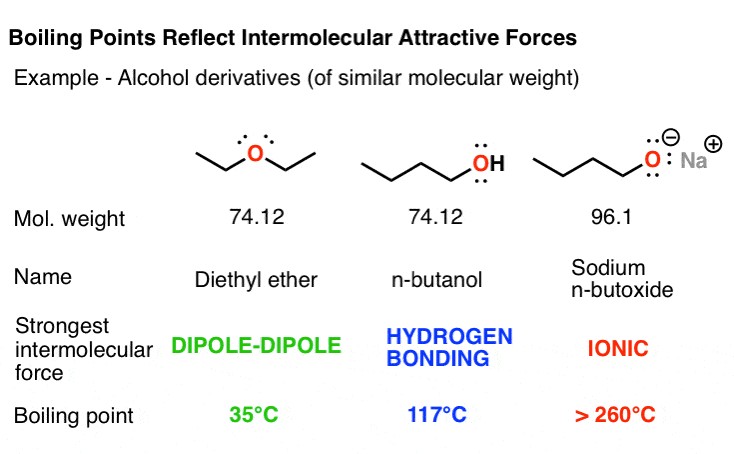
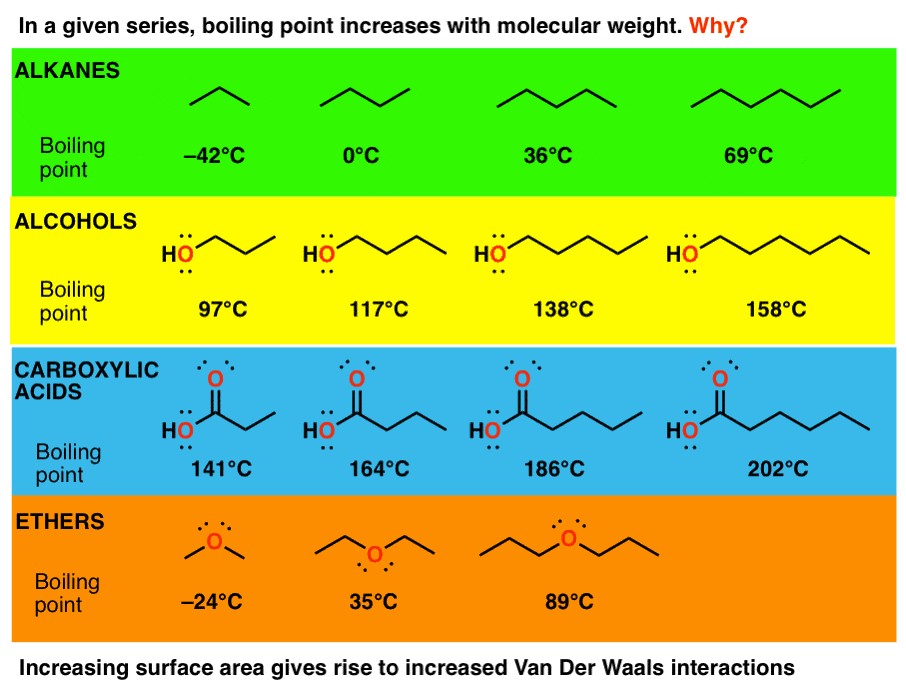
4.1. How do alcohols compare to ethers in terms of boiling points?
Alcohols have significantly higher boiling points than ethers with similar molecular weights. This difference is primarily due to the presence of hydrogen bonding in alcohols. The hydroxyl (-OH) group in alcohols can form strong hydrogen bonds, whereas ethers, lacking a hydrogen atom bonded to an electronegative atom, can only participate in weaker dipole-dipole interactions.
4.2. Why do carboxylic acids have higher boiling points than alcohols?
Carboxylic acids generally have higher boiling points than alcohols due to their ability to form dimers through hydrogen bonding. Two carboxylic acid molecules can form two hydrogen bonds with each other, effectively doubling the intermolecular attraction compared to alcohols, which can only form single hydrogen bonds.
4.3. How do aldehydes and ketones compare to alkanes and alkenes?
Aldehydes and ketones have higher boiling points than alkanes and alkenes with similar molecular weights. This is because aldehydes and ketones possess a carbonyl (C=O) group, which creates a dipole moment and allows for dipole-dipole interactions. Alkanes and alkenes, on the other hand, primarily rely on weaker Van der Waals dispersion forces.
4.4. What is the influence of halides on boiling points?
The presence of halides increases boiling points compared to alkanes due to the dipole-dipole interactions arising from the polar C-X bond (where X is a halogen). The boiling point increases with the size of the halogen atom (F < Cl < Br < I) due to the increasing strength of Van der Waals forces and the greater polarizability of larger halogens.
4.5. How do amines compare to alcohols in terms of boiling points?
Amines can form hydrogen bonds, but these bonds are generally weaker than those formed by alcohols. Oxygen is more electronegative than nitrogen, leading to stronger hydrogen bonds in alcohols. As a result, alcohols typically have higher boiling points than amines with similar molecular weights.
5. Boiling Points and Phase Transitions
Boiling point is the temperature at which a substance changes from a liquid to a gas. Understanding boiling points is essential for predicting and controlling phase transitions in various chemical processes.
5.1. What is the relationship between boiling point and vapor pressure?
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. When the vapor pressure reaches atmospheric pressure, bubbles of vapor form throughout the liquid and rise to the surface, resulting in boiling.
5.2. How does atmospheric pressure affect boiling points?
Boiling points are dependent on atmospheric pressure. At higher altitudes, where atmospheric pressure is lower, liquids boil at lower temperatures. Conversely, at lower altitudes or in pressurized systems, liquids boil at higher temperatures.
5.3. What is the difference between boiling and evaporation?
Boiling and evaporation are both processes by which a liquid turns into a gas, but they occur differently. Evaporation occurs at the surface of a liquid at any temperature, whereas boiling occurs throughout the liquid at a specific temperature (the boiling point). Evaporation is a slower process than boiling.
5.4. How are boiling points used in distillation?
Distillation is a process used to separate liquids with different boiling points. By heating a mixture, the component with the lower boiling point vaporizes first and is then condensed and collected separately. This process is widely used in chemical and industrial applications to purify substances.
5.5. How can boiling points indicate the purity of a substance?
The boiling point of a pure substance is a characteristic property and is relatively constant. Impurities can affect the boiling point, often causing it to increase and broaden the boiling range. Therefore, measuring the boiling point can be an indicator of the purity of a substance.
6. Predicting Boiling Points: Guidelines and Examples
Predicting boiling points involves considering intermolecular forces, molecular weight, and molecular shape. Here are some guidelines and examples:
- Identify the functional groups: Determine the types of intermolecular forces present.
- Assess molecular weight: Larger molecules generally have higher boiling points.
- Evaluate molecular shape: Branched molecules tend to have lower boiling points.
Example 1: Compare the boiling points of ethanol (C2H5OH) and dimethyl ether (CH3OCH3).
- Ethanol has hydrogen bonding.
- Dimethyl ether has dipole-dipole interactions.
- Both have similar molecular weights.
Prediction: Ethanol has a higher boiling point due to hydrogen bonding.
Example 2: Compare the boiling points of n-hexane and 2-methylpentane.
- Both are alkanes with only Van der Waals forces.
- Both have the same molecular weight.
- N-hexane is straight-chain, while 2-methylpentane is branched.
Prediction: N-hexane has a higher boiling point due to its larger surface area.
6.1. What are the key steps in predicting boiling points?
Predicting boiling points involves several key steps:
- Identify Functional Groups: Determine the types of functional groups present in the molecule, as these dictate the types of intermolecular forces that can occur.
- Assess Intermolecular Forces: Evaluate the strength and type of intermolecular forces (Van der Waals, dipole-dipole, hydrogen bonding, ionic) that the molecule can exhibit.
- Consider Molecular Weight: Compare the molecular weights of the substances, as larger molecules generally have higher boiling points due to increased Van der Waals forces.
- Evaluate Molecular Shape: Assess the molecular shape, looking for branching or symmetry, which can reduce the surface area available for intermolecular interactions.
- Compare and Contrast: Compare and contrast all these factors to make a prediction about relative boiling points.
6.2. How can you use functional groups to predict boiling points?
Functional groups determine the types of intermolecular forces that a molecule can exhibit. For example, alcohols and carboxylic acids can form hydrogen bonds, leading to higher boiling points compared to alkanes, which only exhibit Van der Waals forces. By identifying the functional groups, you can predict the relative strength of intermolecular forces and, consequently, the boiling points.
6.3. What role does molecular structure play in predicting boiling points?
Molecular structure, including branching and symmetry, affects the surface area available for intermolecular interactions. Linear molecules have greater surface areas and stronger Van der Waals forces, leading to higher boiling points. Branched molecules have smaller surface areas and weaker intermolecular forces, resulting in lower boiling points.
6.4. How accurate are boiling point predictions?
The accuracy of boiling point predictions depends on the complexity of the molecules and the precision of the data used. For simple molecules within homologous series, predictions can be relatively accurate. However, for complex molecules with multiple functional groups or unusual structures, predictions may be less precise.
6.5. Are there online tools or databases that can assist in predicting boiling points?
Yes, there are several online tools and databases that can assist in predicting boiling points. These resources often use predictive algorithms and extensive data sets to estimate boiling points based on molecular structure and properties. Examples include chemical property calculators and databases like PubChem and ChemSpider.
7. Factors Affecting the Accuracy of Boiling Point Measurements
Several factors can affect the accuracy of boiling point measurements. These include:
- Impurities: Impurities in the sample can alter the boiling point.
- Pressure variations: Changes in atmospheric pressure can affect the boiling point.
- Superheating: The liquid may become hotter than its boiling point without boiling.
- Calibration of equipment: Inaccurate thermometers or other measuring devices can lead to errors.
7.1. How do impurities affect boiling point measurements?
Impurities typically elevate the boiling point and broaden the boiling range of a substance. This phenomenon, known as boiling point elevation, is a colligative property that depends on the concentration of impurity particles in the solution.
7.2. What is superheating, and how does it affect boiling point measurements?
Superheating occurs when a liquid is heated to a temperature slightly above its boiling point without actually boiling. This can happen in smooth, clean containers where there are no nucleation sites for bubble formation. Superheating can lead to an inaccurate boiling point measurement because the liquid may suddenly and violently boil when a disturbance is introduced.
7.3. How does the rate of heating influence boiling point measurements?
The rate of heating can affect the accuracy of boiling point measurements. Heating too quickly can cause the liquid to superheat, leading to inaccurate results. Slow, controlled heating is recommended to ensure that the liquid reaches its true boiling point and maintains equilibrium.
7.4. How important is the calibration of thermometers and other measuring devices?
Accurate calibration of thermometers and other measuring devices is crucial for obtaining reliable boiling point measurements. A poorly calibrated thermometer can introduce systematic errors, leading to significant discrepancies in the measured boiling point.
7.5. What are the best practices for accurate boiling point measurements in a laboratory setting?
Best practices for accurate boiling point measurements include:
- Using a pure sample.
- Calibrating thermometers regularly.
- Heating the liquid slowly and evenly.
- Using boiling chips or a magnetic stirrer to prevent superheating.
- Ensuring accurate pressure measurements.
- Observing the boiling point carefully and recording a range rather than a single temperature.
8. Real-World Applications of Boiling Point Knowledge
Understanding boiling points has numerous practical applications in various fields:
- Chemical industry: Distillation processes rely on boiling point differences to separate and purify chemicals.
- Pharmaceuticals: Boiling points are crucial in drug formulation and purification.
- Food industry: Boiling points are used in cooking, brewing, and food processing.
- Petroleum refining: Crude oil is separated into various fractions based on boiling points.
- Environmental science: Boiling points help in identifying and quantifying volatile organic compounds (VOCs).
8.1. How are boiling points used in the petroleum industry?
In the petroleum industry, fractional distillation is used to separate crude oil into various components based on their boiling points. Crude oil is heated, and the vapors are passed through a distillation column. As the vapors rise, they cool and condense at different levels, separating into fractions like gasoline, kerosene, and lubricating oil, each with different boiling point ranges.
8.2. What role do boiling points play in the food and beverage industry?
Boiling points are critical in the food and beverage industry for processes like cooking, brewing, and distillation of alcoholic beverages. They influence the cooking temperature, the efficiency of extraction processes, and the separation of volatile aroma compounds.
8.3. How are boiling points utilized in the pharmaceutical industry?
In the pharmaceutical industry, boiling points are used in the purification and isolation of drug compounds. Distillation and other separation techniques based on boiling points are essential for producing high-purity pharmaceuticals.
8.4. What is the significance of boiling points in environmental science?
Boiling points are significant in environmental science for identifying and quantifying volatile organic compounds (VOCs) in air and water samples. VOCs are often separated and analyzed using gas chromatography, which relies on boiling point differences to separate the compounds.
8.5. How are boiling points used in the development of new materials?
Boiling points are important in the development of new materials, particularly in the synthesis and processing of polymers and other organic compounds. Understanding the boiling points of reactants and solvents is crucial for optimizing reaction conditions and purification processes.
9. Case Studies: Comparing Boiling Points in Specific Compounds
Several case studies can illustrate the principles of comparing boiling points:
Case Study 1: Comparing the boiling points of n-butane (C4H10) and acetone (CH3COCH3).
- N-butane has only Van der Waals forces; boiling point: -0.5°C.
- Acetone has dipole-dipole interactions and Van der Waals forces; boiling point: 56°C.
Acetone has a significantly higher boiling point due to the presence of dipole-dipole interactions.
Case Study 2: Comparing the boiling points of ethanol (C2H5OH) and diethyl ether (C2H5OC2H5).
- Ethanol has hydrogen bonding; boiling point: 78.37°C.
- Diethyl ether has dipole-dipole interactions; boiling point: 34.6°C.
Ethanol has a higher boiling point due to hydrogen bonding.
Case Study 3: Comparing the boiling points of n-pentane (C5H12) and neopentane (2,2-dimethylpropane).
- N-pentane is straight-chain; boiling point: 36.1°C.
- Neopentane is branched; boiling point: 9.5°C.
N-pentane has a higher boiling point due to its larger surface area.
9.1. Case Study: Alcohols vs. Ethers
Consider ethanol (C2H5OH) and dimethyl ether (CH3OCH3). Ethanol has a hydroxyl group that allows for hydrogen bonding, while dimethyl ether can only participate in dipole-dipole interactions. The boiling point of ethanol is 78.37°C, whereas dimethyl ether boils at -24°C. This stark difference highlights the significant impact of hydrogen bonding on boiling points.
9.2. Case Study: Straight-Chain vs. Branched Alkanes
Compare n-pentane (C5H12) and 2,2-dimethylpropane (neopentane). Both have the same molecular formula, but n-pentane is a straight-chain alkane, while neopentane is highly branched. N-pentane has a boiling point of 36.1°C, while neopentane boils at 9.5°C. The lower boiling point of neopentane is due to its reduced surface area and weaker Van der Waals forces.
9.3. Case Study: Aldehydes and Ketones
Consider propanal (CH3CH2CHO) and propanone (CH3COCH3), also known as acetone. Both compounds have similar molecular weights and exhibit dipole-dipole interactions due to the carbonyl group. Propanal has a boiling point of 48.8°C, while acetone boils at 56°C. The slight difference can be attributed to the shape and polarity differences between the two molecules.
9.4. Case Study: Carboxylic Acids
Acetic acid (CH3COOH) has a significantly higher boiling point (118°C) compared to ethanol (78.37°C), even though they have similar molecular weights. This is because acetic acid can form dimers through hydrogen bonding, effectively doubling the intermolecular attraction.
9.5. Case Study: Haloalkanes
Comparing chloromethane (CH3Cl), bromomethane (CH3Br), and iodomethane (CH3I) demonstrates the effect of increasing halogen size on boiling points. The boiling points increase in the order chloromethane (-24°C), bromomethane (3.6°C), and iodomethane (42°C). This trend is due to the increasing strength of Van der Waals forces with the size of the halogen atom.
10. Common Mistakes to Avoid When Comparing Boiling Points
When comparing boiling points, avoid these common mistakes:
- Ignoring intermolecular forces: Failing to consider the types and strengths of intermolecular forces.
- Overlooking molecular shape: Not accounting for the impact of branching and symmetry.
- Disregarding molecular weight: Neglecting the role of molecular weight, especially in homologous series.
- Assuming all hydrogen bonds are equal: Not recognizing that hydrogen bond strength varies with the electronegativity of the atoms involved.
- Neglecting pressure effects: Forgetting that boiling points are pressure-dependent.
10.1. Ignoring Intermolecular Forces
One of the most common mistakes is failing to consider the types and strengths of intermolecular forces (IMFs) present in a molecule. IMFs play a crucial role in determining boiling points, and neglecting them can lead to inaccurate comparisons. For example, assuming that all molecules with similar molecular weights will have similar boiling points, without considering hydrogen bonding or dipole-dipole interactions, is a mistake.
10.2. Overlooking Molecular Shape
Molecular shape significantly affects the surface area available for intermolecular interactions. Branched molecules have lower boiling points than their straight-chain counterparts due to reduced surface contact. Overlooking this factor can lead to incorrect predictions of boiling points.
10.3. Disregarding Molecular Weight
While intermolecular forces are critical, molecular weight also plays a significant role, especially within homologous series. Larger molecules generally have higher boiling points due to increased Van der Waals forces. Disregarding molecular weight can lead to incorrect comparisons, particularly when comparing molecules with similar functional groups but different sizes.
10.4. Assuming All Hydrogen Bonds Are Equal
Not all hydrogen bonds are created equal. The strength of a hydrogen bond depends on the electronegativity of the atoms involved. For example, hydrogen bonds involving oxygen (as in alcohols and carboxylic acids) are stronger than those involving nitrogen (as in amines). Assuming that all hydrogen bonds have the same impact on boiling points can lead to inaccuracies.
10.5. Neglecting Pressure Effects
Boiling points are pressure-dependent. The standard boiling point is defined at 1 atmosphere of pressure. However, boiling points will vary at different pressures. Neglecting to account for pressure effects can lead to inaccurate comparisons, especially in non-standard conditions.
FAQ: Understanding Boiling Points of Compounds
Q1: What are the primary factors that influence the boiling point of a compound?
A1: The primary factors are intermolecular forces (Van der Waals, dipole-dipole, hydrogen bonding, ionic), molecular weight, and molecular shape.
Q2: How does hydrogen bonding affect boiling point?
A2: Hydrogen bonding significantly increases boiling point due to strong intermolecular attractions.
Q3: Why do branched molecules generally have lower boiling points?
A3: Branched molecules have lower boiling points because branching reduces surface area and weakens Van der Waals forces.
Q4: What is the relationship between molecular weight and boiling point?
A4: Generally, boiling point increases with molecular weight due to increased Van der Waals forces.
Q5: How do impurities affect boiling point measurements?
A5: Impurities typically elevate the boiling point and broaden the boiling range.
Q6: What is superheating, and how can it be prevented?
A6: Superheating is when a liquid is heated above its boiling point without boiling; it can be prevented by using boiling chips or a magnetic stirrer.
Q7: How does atmospheric pressure affect boiling point?
A7: Lower atmospheric pressure decreases boiling point, while higher pressure increases it.
Q8: What are some real-world applications of understanding boiling points?
A8: Applications include distillation in the chemical and petroleum industries, food processing, and pharmaceutical purification.
Q9: How can functional groups be used to predict boiling points?
A9: Functional groups determine the types of intermolecular forces, which influence boiling points; compounds with stronger intermolecular forces have higher boiling points.
Q10: What is the difference between boiling and evaporation?
A10: Boiling occurs throughout the liquid at a specific temperature, while evaporation occurs at the surface at any temperature.
Conclusion: Mastering Boiling Point Comparisons for Informed Decisions
Understanding How To Compare Boiling Points Of Compounds requires a grasp of intermolecular forces, molecular weight, and molecular shape. By considering these factors, you can make accurate predictions and informed decisions in various scientific and industrial applications. Remember to avoid common mistakes and utilize available tools and resources to enhance your understanding. For more in-depth comparisons and detailed analysis, visit COMPARE.EDU.VN, where we provide comprehensive information to assist you in making the best choices. Whether you’re a student, a researcher, or an industry professional, COMPARE.EDU.VN is your go-to source for reliable and objective comparisons.
Need more help with your comparisons? Contact us at:
- Address: 333 Comparison Plaza, Choice City, CA 90210, United States
- Whatsapp: +1 (626) 555-9090
- Website: COMPARE.EDU.VN
Let compare.edu.vn empower you to make the most informed decisions!