The wave and particle natures of light, a concept known as wave-particle duality, present a fascinating paradox in physics, meticulously explored at COMPARE.EDU.VN. Light exhibits properties of both waves, like diffraction and interference, and particles, called photons, each carrying a specific amount of energy. This dual behavior is fundamental to understanding light and its interactions with matter, influencing various technologies from lasers to solar panels. Explore the intricacies of light behavior, photonic nature, and wave characteristics to unlock the secrets of light.
1. Introduction to the Wave-Particle Duality of Light
Light, an essential part of our everyday lives, has intrigued scientists for centuries. One of the most fascinating and perplexing aspects of light is its dual nature: it behaves as both a wave and a particle. This concept, known as wave-particle duality, is a cornerstone of quantum mechanics and has revolutionized our understanding of the universe. COMPARE.EDU.VN provides a comprehensive exploration of this duality, offering insights into the characteristics, experiments, and implications of light’s wave and particle behaviors.
1.1 Understanding the Basic Concepts
Before diving into the comparison, it’s essential to grasp the basic concepts of wave and particle theories of light.
- Wave Theory: This theory describes light as an electromagnetic wave, characterized by its wavelength, frequency, and amplitude. Waves can diffract, interfere, and polarize.
- Particle Theory: This theory describes light as a stream of particles called photons. Each photon carries a specific amount of energy, proportional to the frequency of the light. Particles can be absorbed or emitted.
1.2 Historical Context
The debate over whether light is a wave or a particle dates back to the 17th century.
- Christiaan Huygens proposed the wave theory, explaining phenomena like reflection and refraction.
- Isaac Newton championed the particle theory, arguing that light consists of tiny particles.
It wasn’t until the 20th century that the wave-particle duality was fully recognized and accepted, thanks to groundbreaking experiments and theoretical advancements.
2. The Wave Nature of Light
The wave nature of light is evident in several phenomena, demonstrating its ability to behave like a wave.
2.1 Wave Properties
Light exhibits typical wave behaviors, including:
- Diffraction: The bending of light waves around obstacles or through narrow openings.
- Interference: The superposition of two or more light waves, resulting in constructive (increased amplitude) or destructive (decreased amplitude) interference patterns.
- Polarization: The restriction of light waves to vibrate in a single plane.
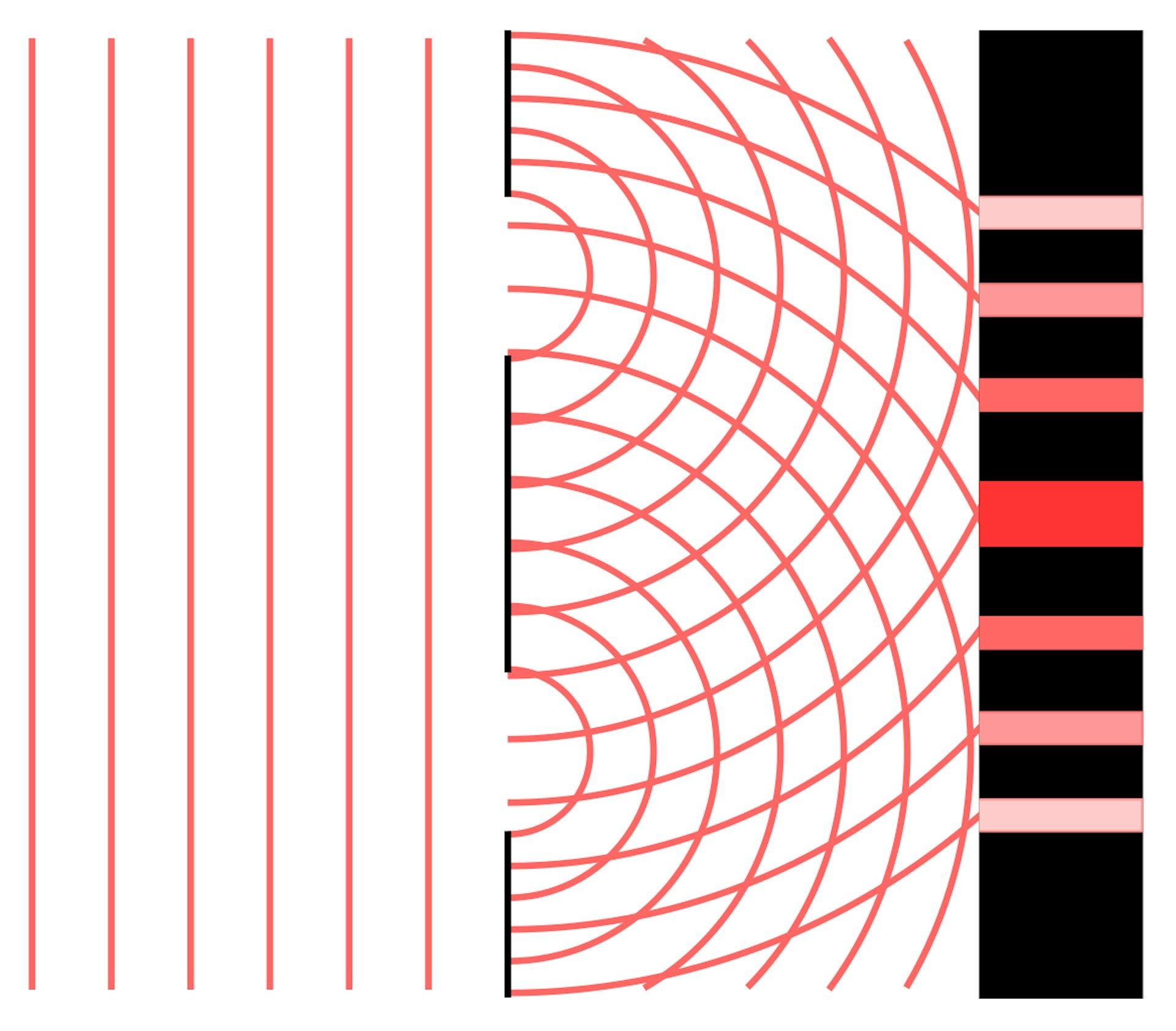
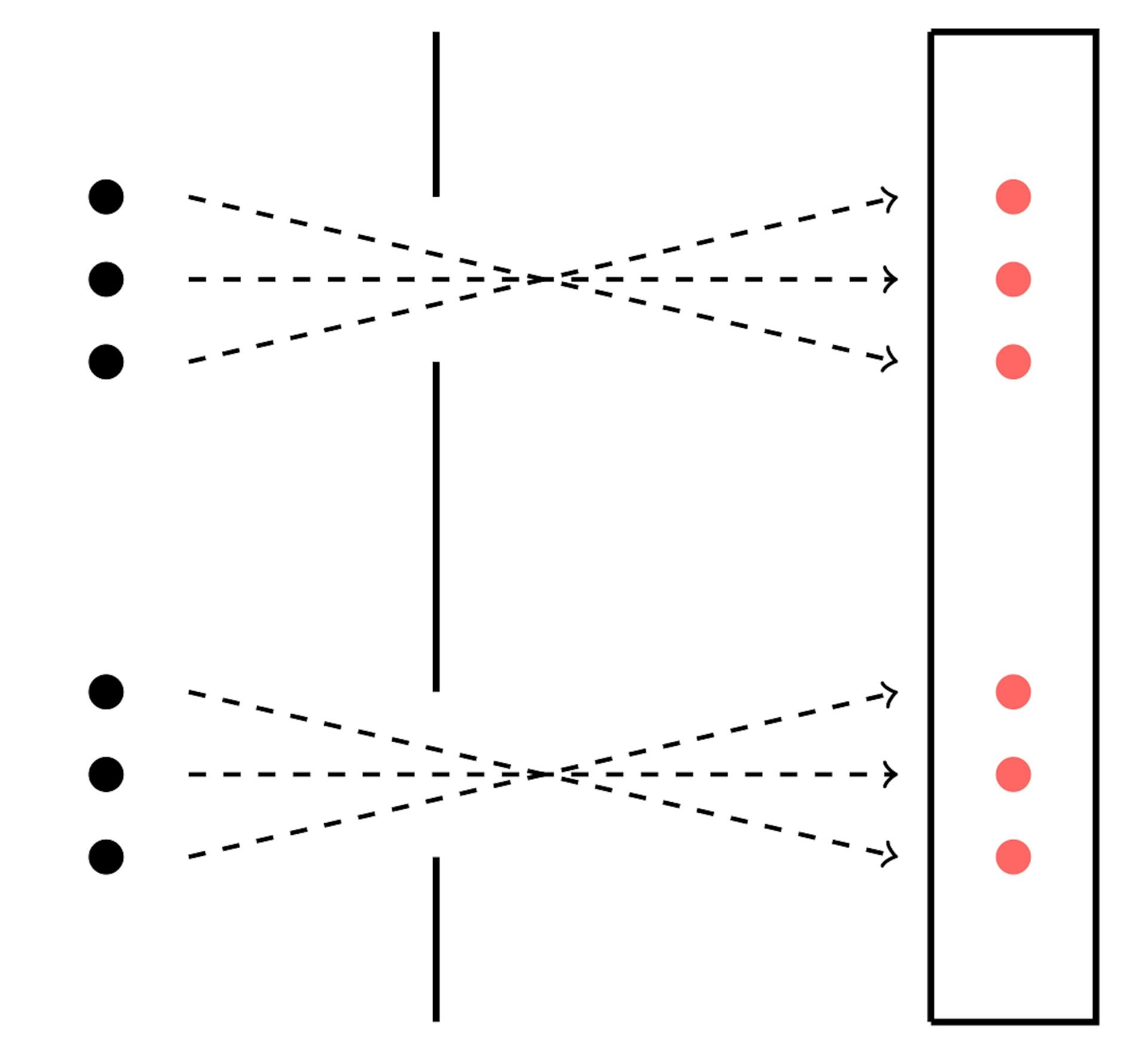
2.2 The Double-Slit Experiment
One of the most compelling pieces of evidence for the wave nature of light is the double-slit experiment.
- When light passes through two narrow slits, it creates an interference pattern on a screen behind the slits.
- This pattern consists of alternating bright and dark fringes, indicating constructive and destructive interference, respectively.
- The double-slit experiment demonstrates that light waves can split and interfere with each other, a behavior unique to waves.
2.3 Mathematical Description of Light Waves
Light waves can be mathematically described using equations that define their properties:
- Wavelength (λ): The distance between two consecutive crests or troughs of a wave.
- Frequency (ν): The number of wave cycles that pass a given point per unit time.
- Speed of Light (c): The constant speed at which light travels in a vacuum (approximately 3.0 x 10^8 meters per second).
The relationship between these properties is given by the equation:
c = λνThis equation shows that the speed of light is the product of its wavelength and frequency.
3. The Particle Nature of Light
The particle nature of light is demonstrated through phenomena that show light behaving as a stream of discrete particles.
3.1 Photons
According to the particle theory, light consists of particles called photons.
- Energy: Each photon carries a specific amount of energy, which is directly proportional to the frequency of the light.
- Momentum: Photons also have momentum, which is related to their energy and speed.
- Massless: Photons are massless particles, meaning they have no rest mass.
3.2 The Photoelectric Effect
The photoelectric effect is a crucial experiment that supports the particle nature of light.
- When light shines on a metal surface, electrons are emitted from the metal.
- The energy of the emitted electrons depends on the frequency of the light, not its intensity.
- This observation cannot be explained by the wave theory of light but is easily explained by the particle theory.
3.3 Einstein’s Explanation
Albert Einstein provided an explanation for the photoelectric effect, which earned him the Nobel Prize in Physics.
- Einstein proposed that light consists of discrete packets of energy (photons).
- When a photon strikes the metal surface, it transfers its energy to an electron.
- If the photon has enough energy, the electron can overcome the binding energy of the metal and be emitted.
3.4 Mathematical Description of Photons
The energy of a photon can be calculated using the equation:
E = hνWhere:
- E is the energy of the photon.
- h is Planck’s constant (approximately 6.626 x 10^-34 joule-seconds).
- ν is the frequency of the light.
This equation shows that the energy of a photon is directly proportional to its frequency.
4. Comparing Wave and Particle Natures of Light
To fully understand the wave-particle duality, it is essential to compare the characteristics, behaviors, and implications of both natures of light.
4.1 Key Differences
Here is a comparison of the key differences between the wave and particle natures of light:
| Feature | Wave Nature | Particle Nature |
|---|---|---|
| Description | Electromagnetic wave | Stream of discrete particles (photons) |
| Characteristics | Wavelength, frequency, amplitude, polarization | Energy, momentum |
| Behavior | Diffraction, interference, refraction | Absorption, emission |
| Experiments | Double-slit experiment, polarization experiments | Photoelectric effect, Compton scattering |
| Mathematical Description | c = λν | E = hν |
| Explanation of Phenomena | Explains wave-like behaviors such as interference patterns and diffraction. | Explains particle-like behaviors such as the emission of electrons in the photoelectric effect. |
| Nature of Light | Light propagates as a continuous wave. | Light consists of discrete packets of energy (photons). |
| Interaction with Matter | Light waves interact with matter through oscillating electric and magnetic fields. | Photons interact with matter by transferring their energy and momentum to individual particles (e.g., electrons). |
| Applications | Optical fibers, radio communication, wave optics | Solar panels, medical imaging, particle physics |
| Limitations | Fails to explain the photoelectric effect and the discrete nature of light absorption and emission. | Fails to explain wave-like behaviors such as interference and diffraction. |

4.2 Strengths and Weaknesses
Both the wave and particle theories have their strengths and weaknesses in explaining the behavior of light:
- Wave Theory:
- Strengths: Explains diffraction, interference, and polarization.
- Weaknesses: Fails to explain the photoelectric effect and the discrete nature of light absorption and emission.
- Particle Theory:
- Strengths: Explains the photoelectric effect and the discrete nature of light absorption and emission.
- Weaknesses: Fails to explain diffraction, interference, and polarization.
4.3 Complementary Nature
The wave and particle natures of light are not mutually exclusive but rather complementary. Light exhibits both wave-like and particle-like behaviors, depending on the experiment or phenomenon being observed.
5. Wave-Particle Duality in Quantum Mechanics
Quantum mechanics provides a framework for understanding the wave-particle duality of light and other quantum entities.
5.1 The de Broglie Hypothesis
In 1924, Louis de Broglie proposed that all matter exhibits wave-particle duality. He suggested that particles, like electrons, also have a wavelength associated with them, given by the equation:
λ = h/p
Where:
- λ is the wavelength of the particle.
- h is Planck’s constant.
- p is the momentum of the particle.
This hypothesis was later confirmed by experiments showing that electrons can also exhibit diffraction and interference, similar to light.
5.2 The Uncertainty Principle
The Heisenberg uncertainty principle is another fundamental concept in quantum mechanics that relates to wave-particle duality.
- The uncertainty principle states that it is impossible to simultaneously know both the position and momentum of a particle with perfect accuracy.
- This principle arises from the wave-like nature of particles and the limitations of measurement in quantum mechanics.
5.3 Quantum Field Theory
Quantum field theory (QFT) provides a more advanced framework for understanding wave-particle duality.
- QFT describes particles as excitations of quantum fields that permeate all of space.
- These fields exhibit both wave-like and particle-like properties, providing a unified description of matter and energy.
6. Practical Applications of Wave-Particle Duality
The wave-particle duality of light has numerous practical applications in various fields, including technology, medicine, and research.
6.1 Lasers
Lasers are devices that produce coherent beams of light with specific wavelengths and frequencies.
- Lasers utilize the wave nature of light to create interference patterns that amplify the light intensity.
- They also rely on the particle nature of light for processes like stimulated emission.
6.2 Solar Panels
Solar panels convert light into electricity using the photoelectric effect.
- Photons from sunlight strike the solar panel, transferring their energy to electrons in the semiconductor material.
- The electrons are then released and flow through an electrical circuit, generating electricity.
6.3 Microscopy
Electron microscopes use the wave nature of electrons to create high-resolution images of tiny objects.
- Electrons are accelerated to high speeds, giving them very short wavelengths.
- These short wavelengths allow the microscope to resolve details that are much smaller than those visible with light microscopes.
6.4 Medical Imaging
Medical imaging techniques like X-rays and PET scans rely on the particle nature of light.
- X-rays are high-energy photons that can penetrate soft tissues, allowing doctors to visualize bones and internal organs.
- PET scans use radioactive isotopes that emit positrons, which annihilate with electrons, producing gamma rays that can be detected and used to create images.
7. Implications for Technology and Research
The wave-particle duality of light continues to influence technological advancements and scientific research.
7.1 Quantum Computing
Quantum computing uses quantum bits (qubits) to perform calculations.
- Qubits can exist in multiple states simultaneously, thanks to the superposition principle, which is related to wave-particle duality.
- This allows quantum computers to perform certain calculations much faster than classical computers.
7.2 Nanotechnology
Nanotechnology involves the manipulation of matter at the atomic and molecular level.
- Understanding the wave-particle duality of electrons is crucial for designing and building nanoscale devices.
- Techniques like electron beam lithography rely on the wave nature of electrons to create precise patterns on surfaces.
7.3 Fundamental Research
The wave-particle duality continues to be a topic of ongoing research in physics.
- Scientists are exploring the fundamental nature of quantum mechanics and the relationship between matter and energy.
- Experiments are being conducted to test the limits of wave-particle duality and to search for new phenomena that challenge our understanding of the universe.
8. Conclusion: Embracing the Duality
The wave-particle duality of light is a profound concept that has transformed our understanding of the universe. Light behaves as both a wave and a particle, depending on the experiment or phenomenon being observed. This duality has practical applications in various fields and continues to inspire new technologies and scientific discoveries. By embracing the duality, we can gain a deeper appreciation for the complexity and beauty of the natural world.
COMPARE.EDU.VN understands the challenges in comparing and contrasting complex concepts. We strive to offer detailed and objective comparisons to empower users in making well-informed decisions. If you’re grappling with the wave-particle duality or any other intricate topic, our platform is here to assist. Explore our extensive resources for in-depth analyses and clear explanations.
9. Call to Action
Ready to dive deeper into the fascinating world of wave-particle duality? At COMPARE.EDU.VN, we offer a wealth of resources to help you understand and compare complex scientific concepts. Whether you’re a student, researcher, or simply curious, our platform provides the tools and information you need to make informed decisions. Visit COMPARE.EDU.VN today and discover the clarity you’ve been searching for. Make informed decisions with confidence using compare.edu.vn. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States or Whatsapp: +1 (626) 555-9090.
10. FAQs About Wave-Particle Duality of Light
Here are some frequently asked questions about the wave-particle duality of light:
1. What is wave-particle duality?
Wave-particle duality is the concept that light and other quantum entities exhibit both wave-like and particle-like properties.
2. What experiments demonstrate the wave nature of light?
The double-slit experiment and polarization experiments demonstrate the wave nature of light.
3. What experiments demonstrate the particle nature of light?
The photoelectric effect and Compton scattering demonstrate the particle nature of light.
4. How are the wave and particle natures of light related?
The wave and particle natures of light are complementary, meaning that light exhibits both behaviors depending on the experiment or phenomenon being observed.
5. What is a photon?
A photon is a discrete packet of energy that is the fundamental particle of light.
6. How is the energy of a photon calculated?
The energy of a photon is calculated using the equation E = hν, where E is the energy, h is Planck’s constant, and ν is the frequency of the light.
7. What is the de Broglie hypothesis?
The de Broglie hypothesis states that all matter exhibits wave-particle duality, meaning that particles also have a wavelength associated with them.
8. What is the uncertainty principle?
The uncertainty principle states that it is impossible to simultaneously know both the position and momentum of a particle with perfect accuracy.
9. What are some practical applications of wave-particle duality?
Practical applications of wave-particle duality include lasers, solar panels, electron microscopy, and medical imaging.
10. How does quantum mechanics explain wave-particle duality?
Quantum mechanics provides a framework for understanding wave-particle duality by describing particles as excitations of quantum fields that exhibit both wave-like and particle-like properties.