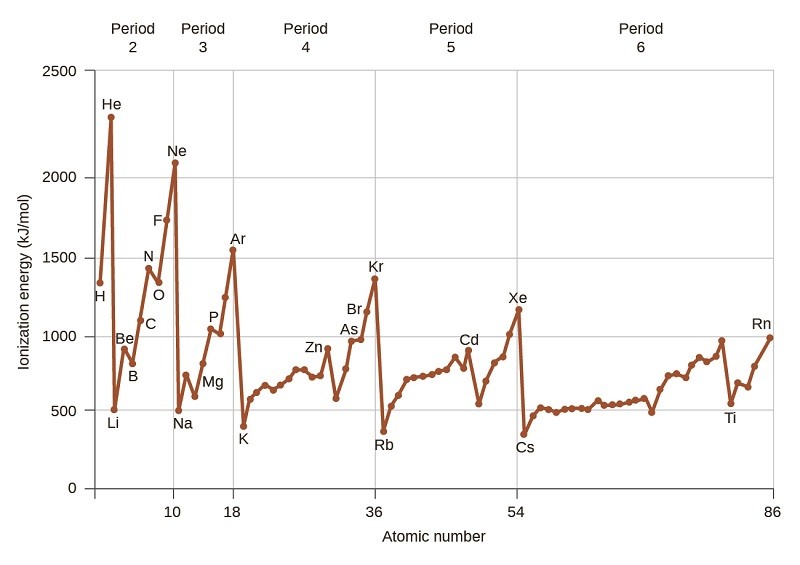
Successive ionization energies always increase because it becomes progressively harder to remove electrons from an increasingly positive ion. This phenomenon is essential for understanding chemical reactivity and electronic structure. Find detailed comparisons and insights at COMPARE.EDU.VN to simplify complex chemical concepts and make informed decisions. Explore ionization trends, energy levels, and electronic configurations with our comprehensive resources.
1. Understanding Ionization Energy
Ionization energy (IE) is the energy required to remove an electron from a gaseous atom or ion. The first ionization energy (IE1) refers to the energy needed to remove the first electron, the second ionization energy (IE2) is for the second electron, and so on.
1.1. What is First Ionization Energy (IE1)?
The first ionization energy (IE1) is the amount of energy required to remove the most loosely bound electron from a neutral gaseous atom in its ground state. For an element X, the process can be represented as:
X(g) → X+(g) + e- IE11.2. What is Second Ionization Energy (IE2)?
The second ionization energy (IE2) is the energy required to remove the second most loosely bound electron from a gaseous ion with a +1 charge. The process can be represented as:
X+(g) → X2+(g) + e- IE21.3. What are Successive Ionization Energies?
Successive ionization energies refer to the energies required to remove subsequent electrons after the first. Each successive ionization energy is higher than the previous one. This trend is crucial in predicting an element’s chemical behavior and the stability of its ions.
2. Factors Affecting Ionization Energy
Several factors influence ionization energy values, including atomic size, nuclear charge, electron shielding, and electron configuration. Understanding these factors helps explain why successive ionization energies increase.
2.1. How Does Atomic Size Impact Ionization Energy?
Atomic size, or atomic radius, significantly affects ionization energy. As atomic size increases, the outermost electrons are farther from the nucleus. This increased distance reduces the electrostatic attraction between the nucleus and the electrons, making it easier to remove an electron. Consequently, ionization energy decreases as atomic size increases.
2.2. How Does Nuclear Charge Impact Ionization Energy?
Nuclear charge refers to the total positive charge of the nucleus due to the presence of protons. A higher nuclear charge results in a stronger attraction between the nucleus and the electrons. This stronger attraction requires more energy to overcome, leading to a higher ionization energy.
2.3. How Does Electron Shielding Impact Ionization Energy?
Electron shielding occurs when inner electrons reduce the effective nuclear charge experienced by the outer electrons. The inner electrons shield the outer electrons from the full attractive force of the nucleus. Effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. The stronger the shielding, the lower the effective nuclear charge, and the easier it is to remove an electron, thus reducing ionization energy.
2.4. How Does Electron Configuration Impact Ionization Energy?
Electron configuration describes the arrangement of electrons within an atom. Atoms with stable electron configurations, such as those with filled or half-filled subshells, exhibit higher ionization energies. Removing an electron from a stable configuration requires more energy due to the increased stability.
3. Why Do Successive Ionization Energies Increase?
Successive ionization energies increase because it becomes progressively harder to remove electrons from an increasingly positive ion. After each electron removal, the remaining electrons experience a greater effective nuclear charge, making them more tightly bound to the nucleus.
3.1. How Does Increased Nuclear Attraction Affect Ionization Energy?
After the removal of the first electron, the resulting ion has a positive charge. This positive charge increases the attraction between the nucleus and the remaining electrons. Removing a second electron from a positively charged ion requires more energy to overcome this increased attraction.
3.2. How Does Reduction in Electron-Electron Repulsion Affect Ionization Energy?
In a neutral atom, electrons repel each other, which partially counteracts the attraction of the nucleus. When an electron is removed, the electron-electron repulsion decreases, allowing the remaining electrons to be more strongly attracted to the nucleus. This increased attraction further raises the ionization energy for subsequent electrons.
3.3. How Does Core Electron Removal Affect Ionization Energy?
Valence electrons, located in the outermost shell, are easier to remove than core electrons, which are closer to the nucleus. Once all valence electrons have been removed, the next electron to be removed is a core electron. Core electrons experience a much stronger effective nuclear charge and are therefore significantly harder to remove. This results in a large jump in ionization energy.
4. Trends in Ionization Energies Across the Periodic Table
Ionization energies exhibit predictable trends across the periodic table. Generally, ionization energy increases across a period (from left to right) and decreases down a group (from top to bottom).
4.1. How Does Ionization Energy Vary Across a Period?
Across a period, the nuclear charge increases while the number of electron shells remains the same. This leads to a stronger attraction between the nucleus and the electrons, resulting in a higher ionization energy.
4.2. How Does Ionization Energy Vary Down a Group?
Down a group, the number of electron shells increases, leading to an increase in atomic size and electron shielding. These factors reduce the effective nuclear charge experienced by the outer electrons, making them easier to remove and thus decreasing ionization energy.
4.3. Exceptions to General Trends
There are some exceptions to the general trends in ionization energies. For example, the ionization energy of boron (B) is less than that of beryllium (Be), and the ionization energy of oxygen (O) is less than that of nitrogen (N). These exceptions are due to the effects of electron configuration and electron pairing.
5. Analyzing Successive Ionization Energy Data
Analyzing successive ionization energy data can provide insights into an element’s electron configuration and chemical properties. Large jumps in ionization energy values indicate the removal of core electrons.
5.1. How to Identify Valence Electrons Using Ionization Energies?
By examining successive ionization energies, one can determine the number of valence electrons in an atom. The ionization energies increase gradually as valence electrons are removed. However, a significant jump in ionization energy indicates the removal of a core electron. The number of electrons removed before this jump corresponds to the number of valence electrons.
5.2. How to Predict the Stability of Ions Using Ionization Energies?
The stability of an ion can be predicted based on its electron configuration and ionization energies. Ions with electron configurations similar to noble gases (filled valence shells) are generally more stable. The energy required to remove additional electrons from these stable ions is significantly higher, indicating their stability.
5.3. Examples of Successive Ionization Energy Analysis
Consider the successive ionization energies for magnesium (Mg) in kJ/mol:
IE1 = 737.7
IE2 = 1451
IE3 = 7733
IE4 = 10540There is a large jump between IE2 and IE3, indicating that magnesium has two valence electrons. This is consistent with magnesium’s electron configuration of [Ne]3s².
6. Successive Ionization Energies of Selected Elements
To further illustrate the concept, let’s examine the successive ionization energies of several elements.
6.1. Potassium (K)
Potassium (K) has one valence electron. Its successive ionization energies (in kJ/mol) are:
IE1 = 418.8
IE2 = 3051.8
IE3 = 4419.6
IE4 = 5876.9
IE5 = 7975.5
IE6 = 9590.6
IE7 = 11343The large jump between IE1 and IE2 indicates that potassium has one valence electron.
6.2. Calcium (Ca)
Calcium (Ca) has two valence electrons. Its successive ionization energies (in kJ/mol) are:
IE1 = 589.8
IE2 = 1145.4
IE3 = 4912.4
IE4 = 6490.6
IE5 = 8153.0
IE6 = 10495.7
IE7 = 12272.9The large jump between IE2 and IE3 indicates that calcium has two valence electrons.
6.3. Scandium (Sc)
Scandium (Sc) has three valence electrons. Its successive ionization energies (in kJ/mol) are:
IE1 = 633.1
IE2 = 1235.0
IE3 = 2388.7
IE4 = 7090.6
IE5 = 8842.9
IE6 = 10679.0
IE7 = 13315.0The large jump between IE3 and IE4 indicates that scandium has three valence electrons.
6.4. Gallium (Ga)
Gallium (Ga) has three valence electrons. Its successive ionization energies (in kJ/mol) are:
IE1 = 578.8
IE2 = 1979.4
IE3 = 2964.6
IE4 = 6180
IE5 = 8298.7
IE6 = 10873.9
IE7 = 13594.8The large jump between IE3 and IE4 indicates that gallium has three valence electrons.
6.5. Germanium (Ge)
Germanium (Ge) has four valence electrons. Its successive ionization energies (in kJ/mol) are:
IE1 = 762.2
IE2 = 1537.5
IE3 = 3302.1
IE4 = 4410.6
IE5 = 9021.4The large jump between IE4 and IE5 indicates that germanium has four valence electrons.
6.6. Arsenic (As)
Arsenic (As) has five valence electrons. Its successive ionization energies (in kJ/mol) are:
IE1 = 944.5
IE2 = 1793.6
IE3 = 2735.5
IE4 = 4836.8
IE5 = 6042.9
IE6 = 12311.5The large jump between IE5 and IE6 indicates that arsenic has five valence electrons.
7. Comparing Ionization Energies: Worked Examples
To solidify understanding, let’s work through some examples comparing ionization energies.
7.1. Example 1: Ranking Ionization Energies
Predict the order of increasing energy for the following processes: IE1 for Al, IE1 for Tl, IE2 for Na, IE3 for Al.
Solution
Removing the 6p¹ electron from Tl is easier than removing the 3p¹ electron from Al because the higher n orbital is farther from the nucleus, so IE1(Tl) 1(Al). Ionizing the third electron from Al²+ requires more energy because the cation Al²+ exerts a stronger pull on the electron than the neutral Al atom, so IE1(Al) 3(Al). The second ionization energy for sodium removes a core electron, which is a much higher energy process than removing valence electrons. Putting this all together, we obtain:
IE1(Tl) < IE1(Al) < IE3(Al) < IE2(Na)7.2. Example 2: Identifying the Element
An element has the following successive ionization energies (in kJ/mol):
IE1 = 577
IE2 = 1820
IE3 = 2750
IE4 = 11600Identify the element.
Solution
The large jump between IE3 and IE4 indicates that the element has three valence electrons. Looking at the periodic table, an element with three valence electrons and a relatively low first ionization energy is aluminum (Al).
7.3. Example 3: Predicting Ion Stability
Which ion is more stable: Na+ or Na2+?
Solution
Sodium (Na) has the electron configuration [Ne]3s¹. The first ionization energy removes the 3s¹ electron, resulting in the Na+ ion with the electron configuration [Ne], which is a stable noble gas configuration. The second ionization energy would remove a core electron from the [Ne] configuration, requiring significantly more energy. Therefore, Na+ is much more stable than Na2+.
8. Applications of Ionization Energies
Ionization energies have various applications in chemistry, including predicting chemical reactivity, understanding bonding, and analyzing electronic structure.
8.1. How Are Ionization Energies Used to Predict Chemical Reactivity?
Ionization energies provide insights into how easily an atom can lose electrons and form positive ions. Elements with low ionization energies tend to be more reactive because they readily lose electrons to form chemical bonds.
8.2. How Are Ionization Energies Used to Understand Bonding?
Ionization energies are crucial in understanding the nature of chemical bonds. Elements with significantly different ionization energies tend to form ionic bonds, where electrons are transferred from one atom to another. Elements with similar ionization energies tend to form covalent bonds, where electrons are shared between atoms.
8.3. How Are Ionization Energies Used to Analyze Electronic Structure?
By analyzing successive ionization energies, chemists can gain insights into the electronic structure of atoms and ions. The number of valence electrons, the stability of ions, and the energy levels of electrons can all be determined through ionization energy measurements.
9. Common Misconceptions About Ionization Energies
Several misconceptions surround ionization energies. Clarifying these misunderstandings is essential for a thorough understanding.
9.1. Misconception 1: Ionization Energy Always Increases Across a Period
While ionization energy generally increases across a period, there are exceptions due to electron configurations. For instance, the ionization energy of oxygen is less than that of nitrogen because removing an electron from oxygen results in a more stable half-filled p subshell.
9.2. Misconception 2: Ionization Energy Is the Same for All Elements in a Group
Ionization energy decreases down a group, so it is not the same for all elements in a group. The outermost electrons are farther from the nucleus and more shielded from the nuclear charge as you move down a group, making them easier to remove.
9.3. Misconception 3: Only the First Ionization Energy Matters
Successive ionization energies provide valuable information about an element’s electronic structure and chemical properties. Analyzing the trends in successive ionization energies helps determine the number of valence electrons and predict the stability of ions.
10. Conclusion: Mastering Ionization Energy Concepts
Understanding how the values of successive ionization energies compare is fundamental to grasping chemical reactivity, electronic structure, and periodic trends. Successive ionization energies increase due to higher effective nuclear charge and reduced electron-electron repulsion, providing key insights into electron configurations and ion stability.
10.1. COMPARE.EDU.VN: Your Resource for Chemical Comparisons
At COMPARE.EDU.VN, we provide comprehensive and objective comparisons to help you make informed decisions. Whether you’re comparing educational resources, products, or services, our platform offers detailed insights and user reviews to guide you.
10.2. Explore More at COMPARE.EDU.VN
Ready to explore more? Visit COMPARE.EDU.VN today to discover detailed comparisons, expert analyses, and user feedback on a wide range of topics. Make informed decisions with confidence, knowing you have access to the best resources available.
10.3. Contact Us
For more information, visit our website at compare.edu.vn or contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. You can also reach us via WhatsApp at +1 (626) 555-9090.
FAQ: Successive Ionization Energies
1. Why does ionization energy increase across a period?
Ionization energy increases across a period due to increasing nuclear charge and decreasing atomic size, which leads to a stronger attraction between the nucleus and the electrons.
2. Why does ionization energy decrease down a group?
Ionization energy decreases down a group due to increasing atomic size and electron shielding, which reduces the effective nuclear charge experienced by the outer electrons.
3. What is the significance of a large jump in successive ionization energies?
A large jump in successive ionization energies indicates the removal of a core electron, which is much harder to remove than a valence electron.
4. How can ionization energies be used to determine the number of valence electrons?
By examining successive ionization energies, the number of electrons removed before a large jump in ionization energy corresponds to the number of valence electrons.
5. What is the difference between IE1 and IE2?
IE1 is the energy required to remove the first electron from a neutral atom, while IE2 is the energy required to remove the second electron from a +1 ion.
6. How does electron configuration affect ionization energy?
Atoms with stable electron configurations, such as filled or half-filled subshells, exhibit higher ionization energies due to the increased stability.
7. What is electron shielding, and how does it affect ionization energy?
Electron shielding is the reduction in effective nuclear charge experienced by outer electrons due to the presence of inner electrons. Greater shielding reduces ionization energy.
8. Why is it harder to remove an electron from a cation than from a neutral atom?
Removing an electron from a cation is harder due to the increased positive charge, which results in a stronger attraction between the nucleus and the remaining electrons.
9. How do successive ionization energies help predict the stability of ions?
Successive ionization energies help predict the stability of ions by indicating the energy required to remove additional electrons. Ions with noble gas configurations are typically more stable.
10. What are some applications of ionization energies in chemistry?
Ionization energies are used to predict chemical reactivity, understand bonding, and analyze the electronic structure of atoms and ions.