The pH scale measures how acidic or basic a solution is. It ranges from 0 to 14, with 7 representing neutral. But how do the numbers on this scale relate to each other? Understanding this logarithmic scale is key to grasping the significant differences between each pH level.
Deciphering the pH Scale: A Logarithmic Relationship
The pH scale isn’t linear; it’s logarithmic. This means each whole number change on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and 100 times more acidic than a solution with a pH of 6. Conversely, a pH of 9 is ten times more basic than a pH of 8. This exponential relationship highlights the dramatic shift in chemical properties with even small changes in pH values.
What Does pH Measure? Hydrogen Ion Concentration
pH actually stands for “potential of hydrogen” or “power of hydrogen.” It measures the concentration of hydrogen ions (H+) in a solution. Acids release H+ ions when dissolved in water, while bases release hydroxide ions (OH-). The higher the H+ concentration, the lower the pH, and the more acidic the solution. The reverse is true for bases: higher OH- concentration leads to higher pH and increased alkalinity.
pH Scale in Action: From Strong Acids to Strong Bases
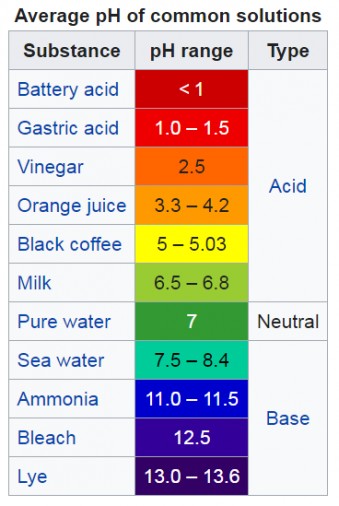
The pH scale spans a wide range of substances, from highly corrosive acids to strong alkalis.
- Strong Acids (pH 0-3): Substances like battery acid (pH 0) and hydrochloric acid (pH 1) have extremely high H+ concentrations and are highly corrosive.
- Weak Acids (pH 4-6): Common examples include coffee (pH 5) and rain water (slightly acidic due to dissolved carbon dioxide).
- Neutral (pH 7): Pure water has a neutral pH of 7, representing a balance between H+ and OH- ions.
- Weak Bases (pH 8-10): Baking soda (pH 9) and seawater (pH 8) are examples of mildly alkaline solutions.
- Strong Bases (pH 11-14): Substances like bleach (pH 13) and drain cleaner (pH 14) possess high OH- concentrations and are caustic.
pH Measurement: Theory and Practice
Understanding the theoretical underpinnings of pH involves the concept of the dissociation constant, which indicates how readily an acid or base releases its ions in water. Strong acids dissociate completely, while weak acids only partially dissociate. This impacts how pH is calculated, especially for weak acids and bases.
In practice, pH is measured using pH meters or indicators. pH meters provide precise numerical readings, while indicators change color depending on the pH of the solution, offering a visual but less precise measurement.
The Importance of pH: Applications Across Diverse Fields
pH measurement is crucial in various fields:
- Environmental Monitoring: Assessing water and soil quality to protect ecosystems.
- Biological Systems: Maintaining the delicate pH balance necessary for life processes in organisms.
- Industrial Processes: Controlling reactions and ensuring product quality in food production, water treatment, and chemical manufacturing.
Conclusion
The pH scale provides a vital framework for understanding and quantifying the acidity and basicity of solutions. Its logarithmic nature highlights the significant differences between seemingly small numerical changes. From everyday life to scientific research and industrial applications, grasping the meaning behind the numbers on the pH scale is essential for numerous purposes.