Hydrolysis and dehydration synthesis are vital chemical processes essential for life, with hydrolysis breaking down polymers by adding water and dehydration synthesis forming polymers by removing water, and this article at COMPARE.EDU.VN will provide you with a comprehensive comparison of these two key reactions. By grasping their nuances, you’ll better understand how biomolecules are constructed and deconstructed, enabling you to analyze biological processes like digestion and cellular respiration with greater clarity. Delve into the energetic aspects, enzyme involvement, and biological significance of both processes to enhance your knowledge.
1. Understanding Hydrolysis
Hydrolysis, derived from the Greek words “hydro” (water) and “lysis” (to split), is a chemical reaction where water is used to break down a compound. In the context of biochemistry, hydrolysis is crucial for the degradation of polymers into their constituent monomers. This process involves the addition of a water molecule to break the covalent bond that holds the monomers together.
1.1. The Mechanism of Hydrolysis
The basic mechanism of hydrolysis involves a water molecule attacking the bond between two monomers in a polymer. The water molecule splits, with one part (H+) attaching to one monomer and the other part (OH-) attaching to the adjacent monomer. This addition of water breaks the bond and separates the monomers.
For example, consider the hydrolysis of a disaccharide like sucrose into glucose and fructose. The reaction can be represented as:
Sucrose + H2O → Glucose + FructoseIn this reaction, the glycosidic bond linking glucose and fructose is broken by the addition of water.
1.2. Types of Hydrolysis Reactions
Hydrolysis reactions can be categorized based on the type of molecule being broken down. Some common types include:
- Hydrolysis of Carbohydrates: Breaking down polysaccharides (like starch and glycogen) into monosaccharides (like glucose).
- Hydrolysis of Proteins: Breaking down polypeptides into amino acids.
- Hydrolysis of Lipids: Breaking down triglycerides into glycerol and fatty acids.
- Hydrolysis of Nucleic Acids: Breaking down DNA and RNA into nucleotides.
1.3. Enzymes in Hydrolysis
Enzymes play a critical role in catalyzing hydrolysis reactions in biological systems. These enzymes, known as hydrolases, lower the activation energy required for the reaction to occur, thereby speeding up the process. Specific enzymes are involved in the hydrolysis of different types of molecules:
- Amylases: Hydrolyze carbohydrates.
- Proteases: Hydrolyze proteins.
- Lipases: Hydrolyze lipids.
- Nucleases: Hydrolyze nucleic acids.
1.4. Importance of Hydrolysis in Biological Systems
Hydrolysis is vital for several biological processes:
- Digestion: Hydrolysis breaks down complex food molecules into smaller, absorbable units in the digestive system. For example, amylases in saliva and the small intestine hydrolyze starch into glucose.
- Cellular Respiration: Hydrolysis provides the necessary monomers (e.g., glucose) that are then used in cellular respiration to produce energy.
- Nutrient Mobilization: Hydrolysis releases stored nutrients from polymers, such as glycogen in the liver being hydrolyzed to release glucose into the bloodstream.
- Waste Removal: Hydrolysis helps break down complex waste molecules into simpler forms that can be excreted.
2. Understanding Dehydration Synthesis
Dehydration synthesis, also known as condensation, is the process of joining two molecules together following the removal of a water molecule. This process is fundamental in the formation of polymers from monomers.
2.1. The Mechanism of Dehydration Synthesis
In dehydration synthesis, a hydroxyl group (OH) is removed from one monomer and a hydrogen atom (H) is removed from the other. These combine to form a water molecule (H2O), and a new covalent bond is formed between the two monomers.
For example, the formation of a disaccharide like maltose from two glucose molecules involves dehydration synthesis:
Glucose + Glucose → Maltose + H2OIn this reaction, a water molecule is removed, and a glycosidic bond is formed between the two glucose molecules.
2.2. Types of Dehydration Synthesis Reactions
Similar to hydrolysis, dehydration synthesis reactions can be categorized based on the type of molecule being formed:
- Formation of Carbohydrates: Monosaccharides combine to form disaccharides and polysaccharides.
- Formation of Proteins: Amino acids combine to form peptides and proteins.
- Formation of Lipids: Glycerol and fatty acids combine to form triglycerides.
- Formation of Nucleic Acids: Nucleotides combine to form DNA and RNA.
2.3. Enzymes in Dehydration Synthesis
Enzymes also play a crucial role in catalyzing dehydration synthesis reactions. These enzymes facilitate the formation of covalent bonds between monomers while ensuring that the reaction occurs efficiently.
- Glycosyltransferases: Catalyze the formation of glycosidic bonds in carbohydrates.
- Peptidyltransferases: Catalyze the formation of peptide bonds in proteins.
- Acyltransferases: Catalyze the formation of ester bonds in lipids.
- Polymerases: Catalyze the formation of phosphodiester bonds in nucleic acids.
2.4. Importance of Dehydration Synthesis in Biological Systems
Dehydration synthesis is essential for:
- Polymer Formation: It is the primary mechanism by which monomers are assembled into polymers, forming complex biological molecules.
- Storage of Energy: Dehydration synthesis allows organisms to store energy in the form of polysaccharides (like starch and glycogen) and lipids (like triglycerides).
- Structural Support: Proteins formed through dehydration synthesis provide structural support in cells and tissues.
- Genetic Information: DNA and RNA, formed through dehydration synthesis, carry genetic information essential for life.
3. How Do Hydrolysis and Dehydration Synthesis Compare?
To effectively compare hydrolysis and dehydration synthesis, it’s important to look at their mechanisms, roles, energy requirements, and enzyme involvement.
3.1. Key Differences
| Feature | Hydrolysis | Dehydration Synthesis |
|---|---|---|
| Definition | Breaking down polymers into monomers by adding water. | Joining monomers to form polymers by removing water. |
| Mechanism | Water molecule splits and is added to monomers, breaking the bond. | Water molecule is removed as a new bond is formed between monomers. |
| Role | Degradation of complex molecules, digestion, waste removal. | Formation of complex molecules, energy storage, structural support. |
| Energy | Releases energy (exergonic). | Requires energy (endergonic). |
| Enzyme Type | Hydrolases (e.g., amylases, proteases, lipases). | Transferases and polymerases (e.g., glycosyltransferases, peptidyltransferases). |
| Overall Result | Polymer + H2O → Monomers | Monomers → Polymer + H2O |
| Examples | Digestion of starch into glucose, breakdown of proteins into amino acids. | Formation of proteins from amino acids, synthesis of glycogen from glucose. |
3.2. Complementary Processes
Hydrolysis and dehydration synthesis are complementary processes in biological systems. While hydrolysis breaks down polymers into their constituent monomers, dehydration synthesis builds polymers from monomers. These two processes work in tandem to maintain the balance of molecules within cells and organisms.
For example, during digestion, hydrolysis breaks down complex carbohydrates into glucose. The glucose molecules are then used in cellular respiration to produce energy. If there is excess glucose, dehydration synthesis is used to form glycogen for storage in the liver and muscles.
3.3. Energetic Considerations
Hydrolysis is an exergonic reaction, meaning it releases energy. This is because the bonds formed between the monomers and water molecules are more stable than the bonds in the original polymer.
Dehydration synthesis, on the other hand, is an endergonic reaction, meaning it requires energy. This is because energy is needed to form the new covalent bond between monomers and to remove the water molecule. This energy is typically supplied by ATP (adenosine triphosphate). According to research from the University of California, Berkeley, the coupling of ATP hydrolysis with dehydration synthesis reactions ensures that these anabolic processes are thermodynamically favorable.
3.4. Role in Maintaining Homeostasis
The balance between hydrolysis and dehydration synthesis is crucial for maintaining homeostasis in biological systems. Homeostasis refers to the ability of an organism to maintain a stable internal environment despite changes in external conditions.
For example, the concentration of glucose in the blood is tightly regulated through the balance of glycogen synthesis (dehydration synthesis) and glycogen breakdown (hydrolysis). After a meal, when blood glucose levels are high, insulin stimulates the synthesis of glycogen in the liver and muscles. During fasting, when blood glucose levels are low, glucagon stimulates the breakdown of glycogen into glucose.
4. Examples of Hydrolysis and Dehydration Synthesis in Biological Systems
To further illustrate the comparison between hydrolysis and dehydration synthesis, let’s consider specific examples in biological systems.
4.1. Carbohydrates
- Hydrolysis: The digestion of starch in the small intestine involves the hydrolysis of glycosidic bonds by amylase, producing glucose monomers.
- Dehydration Synthesis: The formation of glycogen from glucose monomers in the liver involves the dehydration synthesis of glycosidic bonds, storing energy for later use.
4.2. Proteins
- Hydrolysis: The breakdown of proteins in the stomach involves the hydrolysis of peptide bonds by pepsin, producing amino acids.
- Dehydration Synthesis: The synthesis of proteins from amino acids on ribosomes involves the dehydration synthesis of peptide bonds, building complex protein structures.
4.3. Lipids
- Hydrolysis: The digestion of triglycerides in the small intestine involves the hydrolysis of ester bonds by lipases, producing glycerol and fatty acids.
- Dehydration Synthesis: The formation of triglycerides from glycerol and fatty acids in adipose tissue involves the dehydration synthesis of ester bonds, storing energy in the form of fat.
4.4. Nucleic Acids
- Hydrolysis: The breakdown of DNA and RNA by nucleases involves the hydrolysis of phosphodiester bonds, producing nucleotides.
- Dehydration Synthesis: The synthesis of DNA and RNA during replication and transcription involves the dehydration synthesis of phosphodiester bonds, forming the nucleic acid polymers.
5. Implications for Health and Disease
Understanding hydrolysis and dehydration synthesis is crucial not only for comprehending basic biological processes but also for understanding various health conditions and diseases.
5.1. Digestive Disorders
Digestive disorders such as lactose intolerance and celiac disease involve issues with hydrolysis. Lactose intolerance is caused by a deficiency in the enzyme lactase, which hydrolyzes lactose into glucose and galactose. Celiac disease involves an immune reaction to gluten, a protein in wheat, barley, and rye, which impairs the hydrolysis and absorption of nutrients in the small intestine.
5.2. Metabolic Disorders
Metabolic disorders such as glycogen storage diseases involve issues with both hydrolysis and dehydration synthesis. These diseases are caused by defects in enzymes involved in the synthesis or breakdown of glycogen, leading to abnormal accumulation or depletion of glycogen in the liver and muscles.
5.3. Cancer
Cancer cells often exhibit altered rates of hydrolysis and dehydration synthesis. For example, cancer cells may increase the rate of protein synthesis (dehydration synthesis) to support rapid growth and proliferation. Additionally, they may alter the hydrolysis of extracellular matrix components to facilitate invasion and metastasis.
5.4. Aging
Aging is associated with a decline in the efficiency of various biological processes, including hydrolysis and dehydration synthesis. For example, the ability to synthesize proteins may decrease with age, leading to muscle loss and other age-related changes.
6. Experimental Approaches to Studying Hydrolysis and Dehydration Synthesis
Researchers use a variety of experimental approaches to study hydrolysis and dehydration synthesis in biological systems.
6.1. Enzyme Assays
Enzyme assays are used to measure the activity of enzymes involved in hydrolysis and dehydration synthesis. These assays typically involve measuring the rate at which a substrate is converted into a product, or vice versa.
6.2. Spectrophotometry
Spectrophotometry is used to measure the concentration of molecules involved in hydrolysis and dehydration synthesis. This technique involves measuring the absorbance or transmittance of light through a sample, which can be used to determine the concentration of specific molecules.
6.3. Mass Spectrometry
Mass spectrometry is used to identify and quantify the molecules involved in hydrolysis and dehydration synthesis. This technique involves ionizing molecules and then measuring their mass-to-charge ratio, which can be used to identify and quantify specific molecules.
6.4. Isotope Labeling
Isotope labeling is used to trace the fate of atoms during hydrolysis and dehydration synthesis. This technique involves using isotopes of atoms, such as deuterium (2H) or carbon-13 (13C), to label molecules and then tracking their movement during the reaction.
6.5. Genetic Manipulation
Genetic manipulation techniques, such as gene knockout and gene overexpression, are used to study the role of specific enzymes and proteins in hydrolysis and dehydration synthesis. These techniques involve altering the expression of specific genes and then observing the effects on the biological process of interest.
7. Recent Advances in Understanding Hydrolysis and Dehydration Synthesis
Recent research has provided new insights into the mechanisms and regulation of hydrolysis and dehydration synthesis.
7.1. Structural Biology
Structural biology techniques, such as X-ray crystallography and cryo-electron microscopy, have provided detailed structural information about the enzymes involved in hydrolysis and dehydration synthesis. This information has helped researchers understand how these enzymes catalyze reactions at the molecular level. According to a study published in Nature Structural & Molecular Biology, high-resolution structures of glycosyltransferases have revealed the precise interactions between the enzyme, substrate, and nucleotide sugar donor, providing insights into the mechanism of glycosidic bond formation.
7.2. Systems Biology
Systems biology approaches, such as metabolic flux analysis and network modeling, have provided a comprehensive view of the interactions between hydrolysis and dehydration synthesis in metabolic pathways. These approaches have helped researchers understand how these processes are regulated and how they contribute to overall cellular metabolism.
7.3. Synthetic Biology
Synthetic biology approaches have been used to engineer new enzymes and pathways for hydrolysis and dehydration synthesis. This has potential applications in areas such as biofuel production and the synthesis of pharmaceuticals.
7.4. Personalized Medicine
Personalized medicine approaches are being developed to tailor treatments for diseases based on an individual’s genetic makeup and metabolic profile. Understanding the variations in hydrolysis and dehydration synthesis among individuals can help in the development of personalized therapies for digestive disorders, metabolic disorders, and cancer.
8. Future Directions
Future research in hydrolysis and dehydration synthesis is likely to focus on several key areas:
8.1. Developing New Inhibitors and Activators
Developing new inhibitors and activators of enzymes involved in hydrolysis and dehydration synthesis could lead to new treatments for a variety of diseases. For example, inhibitors of enzymes involved in protein synthesis could be used to treat cancer, while activators of enzymes involved in glycogen synthesis could be used to treat diabetes.
8.2. Understanding the Role of Non-Coding RNAs
Non-coding RNAs, such as microRNAs and long non-coding RNAs, have been shown to play a role in regulating gene expression and protein synthesis. Future research is likely to explore the role of non-coding RNAs in regulating hydrolysis and dehydration synthesis.
8.3. Investigating the Impact of Environmental Factors
Environmental factors, such as diet and exposure to toxins, can affect the rates of hydrolysis and dehydration synthesis. Future research is likely to investigate the impact of these factors on human health and disease.
8.4. Applying Advanced Imaging Techniques
Advanced imaging techniques, such as super-resolution microscopy and in vivo imaging, can provide new insights into the spatial and temporal dynamics of hydrolysis and dehydration synthesis in cells and tissues. These techniques can help researchers understand how these processes are coordinated and regulated in complex biological systems.
9. Conclusion
Hydrolysis and dehydration synthesis are fundamental chemical reactions that play essential roles in biological systems. Hydrolysis breaks down polymers into monomers by adding water, while dehydration synthesis forms polymers from monomers by removing water. These processes are complementary and are crucial for maintaining homeostasis, storing energy, providing structural support, and carrying genetic information. Understanding these processes is vital for comprehending basic biological functions and for understanding the causes and treatments of various diseases. For more in-depth comparisons and analyses, visit COMPARE.EDU.VN, where you can explore a wide range of topics and make informed decisions.
Understanding the differences between these two processes—hydration and condensation—allows for a better understanding of biochemistry. This comparison involves the energetic aspects, enzyme involvement, and significance to biological processes. Use this knowledge to help analyze the reactions and processes that occur in the body.
Are you struggling to compare complex biological processes? Do you need help understanding how hydrolysis and dehydration synthesis impact your health? Visit COMPARE.EDU.VN for comprehensive comparisons and expert insights. Our detailed analyses make it easy to understand complex topics and make informed decisions. Don’t stay confused – explore COMPARE.EDU.VN today and discover the clarity you need.
10. Frequently Asked Questions (FAQ)
1. What is the primary difference between hydrolysis and dehydration synthesis?
Hydrolysis breaks down polymers by adding water, whereas dehydration synthesis forms polymers by removing water.
2. Why are enzymes important in hydrolysis and dehydration synthesis?
Enzymes act as catalysts, speeding up the reactions by lowering the activation energy required for the processes to occur.
3. How does hydrolysis contribute to digestion?
Hydrolysis breaks down complex food molecules into smaller, absorbable units, such as breaking down starch into glucose.
4. What role does dehydration synthesis play in energy storage?
Dehydration synthesis forms large storage molecules like glycogen from glucose, storing energy for later use.
5. Are hydrolysis and dehydration synthesis exergonic or endergonic reactions?
Hydrolysis is exergonic (releases energy), while dehydration synthesis is endergonic (requires energy).
6. How do these processes help maintain homeostasis?
They work in tandem to maintain a stable internal environment by balancing the breakdown and synthesis of molecules.
7. Can you give an example of a digestive disorder related to hydrolysis?
Lactose intolerance, where the enzyme lactase is deficient, impairing the hydrolysis of lactose.
8. What is the significance of dehydration synthesis in protein formation?
Dehydration synthesis links amino acids together to form proteins, essential for structural support and various cellular functions.
9. How does structural biology contribute to understanding these processes?
Structural biology provides detailed molecular-level insights into how enzymes catalyze hydrolysis and dehydration synthesis reactions.
10. Where can I find more comprehensive comparisons of biological processes?
Visit COMPARE.EDU.VN for detailed analyses and expert insights on various topics, helping you make informed decisions.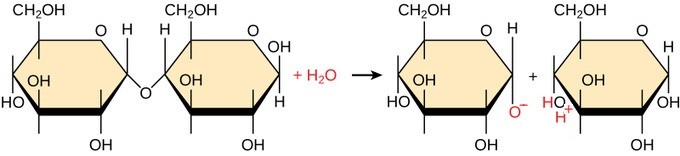 Hydrolysis reaction generating un-ionized products
Hydrolysis reaction generating un-ionized products
Contact Us
For further inquiries or assistance, please reach out to us:
- Address: 333 Comparison Plaza, Choice City, CA 90210, United States
- WhatsApp: +1 (626) 555-9090
- Website: compare.edu.vn

