Introduction
The intricate complexity of the human brain poses significant challenges to understanding its development and disorders using traditional model organisms. This complexity underscores the urgent need for robust in vitro models that can faithfully replicate human brain development. This study introduces a groundbreaking human pluripotent stem cell-derived 3D organoid culture system, termed cerebral organoids, capable of developing diverse and interconnected brain regions. These organoids feature a cerebral cortex with organized progenitor zones that generate mature cortical neuron subtypes, mirroring key aspects of human cortical development, including the presence of outer radial glial (oRG) stem cells. Crucially, unlike mouse models which often fall short, cerebral organoids effectively model human cortical development and disease. This is highlighted by our successful recapitulation of microcephaly, a neurodevelopmental disorder notoriously difficult to model in mice, using RNAi and patient-specific induced pluripotent stem cells (iPSCs). We demonstrate that patient-derived organoids exhibit premature neuronal differentiation, a defect that potentially underlies the disease phenotype. Our findings establish 3D cerebral organoids as a powerful in vitro platform that more accurately reflects the intricacies of human brain development and disease compared to traditional mouse models.
Mammalian brain development commences with the expansion of the neuroepithelium, giving rise to radial glia (RG) [1]. These RGs, located within the ventricular zone (VZ), divide at the apical surface to produce neurons and intermediate progenitors (IPs). IPs then populate the adjacent subventricular zone (SVZ), while neurons migrate through the intermediate zone (IZ) to form specific layers within the cortical plate (CP). Human brain development exhibits a markedly more elaborate organization of progenitor zones compared to rodents. In humans, the SVZ is further divided by an inner fiber layer (IFL) into the inner (ISVZ) and outer SVZ (OSVZ) [2]. The OSVZ represents a distinct progenitor layer, rich in IPs and a unique stem cell type, outer radial glia (oRG) [3, 4], which are only minimally present in rodents [5]. Notably, both the IFL and OSVZ are completely absent in mice [6]. These fundamental differences in progenitor zone organization are critical and contribute to the significant expansion of neuronal output and brain size observed in humans [7, 8].
Primary microcephaly (MCPH), a neurodevelopmental disorder characterized by a significantly reduced brain size [9], has been linked to autosomal recessive mutations in genes encoding proteins associated with the mitotic spindle apparatus [10]. Historically, research into MCPH pathogenesis has relied heavily on mouse models. However, mouse models with mutations in several MCPH-related genes [11-14] have failed to fully replicate the severe reduction in brain size observed in human patients. This limitation highlights a critical gap in our ability to study and understand human-specific neurodevelopmental disorders using mouse models.
Given the substantial developmental disparities between mice and humans, in vitro models that accurately reflect human brain development hold immense potential. While significant strides have been made in in vitro modeling of whole organ development for systems like the intestine [15], pituitary [16], and retina [17, 18], a comprehensive 3D culture model for the developing brain as a whole has remained elusive. Previous research has explored in vitro models of isolated neural tissues [19-23], including dorsal cerebral cortical tissue, which have shown promise in replicating early developmental aspects. However, these models have not fully captured later developmental events such as the formation of discrete cortical layers with inside-out organization, nor have they consistently recapitulated human-specific characteristics like the presence of oRG cells and the unique organization of progenitor zones, features that distinguish human brain development from that of mice.
Here, we introduce a 3D culture system for generating brain tissue in vitro. These cerebral organoids develop diverse regional identities, organized as distinct domains that can influence each other. Importantly, cerebral cortical regions within these organoids exhibit an organization remarkably similar to the developing human brain in early stages, including a significant oRG population. Furthermore, cortical neurons within organoids mature into various pyramidal subtypes with some spatial separation. Finally, and critically, we demonstrate the utility of these organoids for disease modeling by using patient-derived iPSCs and shRNA to model CDK5RAP2-dependent microcephaly, a disorder that has proven challenging to model effectively in mouse models. This highlights the superior translational potential of Cerebral Organoids Compared To Mouse Models for studying human neurodevelopmental disorders.
Results
Generation of Cerebral Organoids: Mimicking Human Brain Development In Vitro
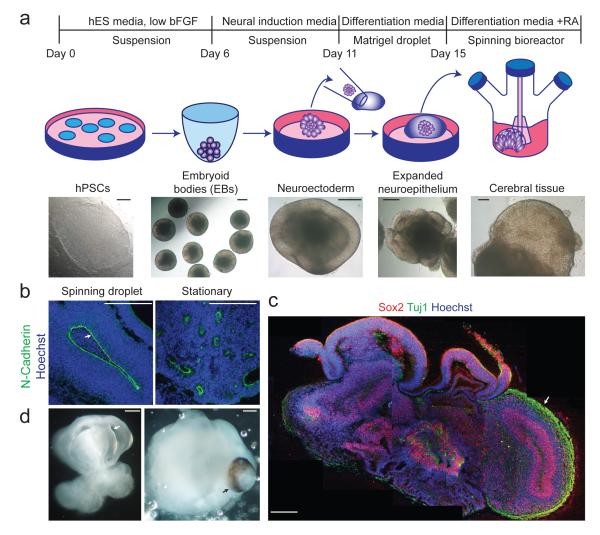
Recent advancements in in vitro modeling of organ systems have demonstrated the remarkable self-organizing capabilities of pluripotent stem cells to form complete tissues [15, 18]. Building on this concept, we developed a protocol that, in contrast to methods relying on patterning growth factors, focuses on optimizing growth conditions and creating an environment conducive to intrinsic developmental cues. We began with a modified approach to generate neuroectoderm from embryoid bodies [24]. These neuroectodermal tissues were then maintained in 3D culture and embedded in Matrigel droplets, providing a scaffold for complex tissue growth. These Matrigel droplets were subsequently transferred to a spinning bioreactor to enhance nutrient absorption (Figure 1a). This innovative method facilitated the rapid development of brain tissues, which we termed cerebral organoids. Neural identity emerged within just 8-10 days, and defined brain regions formed within 20-30 days.
Figure 1. Description of cerebral organoid culture system.
a, Schematic of the cerebral organoid culture system, detailing each stage of the process. Representative images illustrate each developmental stage. b, Neuroepithelial tissues, generated via this method (left panel), exhibit large fluid-filled cavities and typical apical localization of neural N-cadherin (arrow). These tissues were larger and more continuous compared to tissues grown in stationary suspension without Matrigel (right panel). c, Sectioning and immunohistochemistry reveal complex morphology with heterogeneous regions containing neural progenitors (Sox2, red) and neurons (Tuj1, green) (arrow). d, Low magnification bright field images showing fluid-filled cavities resembling ventricles (white arrow) and retina tissue, indicated by retinal pigmented epithelium (black arrow). Scale bars: 200 μm.
At early stages (15-20 days), cerebral organoids developed large, continuous neuroepithelia enclosing a fluid-filled cavity reminiscent of a ventricle, displaying characteristic apical localization of neural-specific N-cadherin (Figure 1b). Furthermore, the neuroepithelium was more expansive and continuous compared to tissues generated using a stationary culture approach [20], which instead formed aggregates of small rosette-like neuroepithelia (Figure 1b, Extended Data Figure 1a).
While tissues reached their maximum size by approximately two months, cerebral organoids matured into large (up to 4 mm in diameter), complex, and heterogeneous tissues capable of surviving indefinitely (currently up to 10 months) when maintained in a spinning bioreactor. Histological and gross morphological analyses revealed regions resembling cerebral cortex, choroid plexus, retina, and meninges (Figure 1c,d, Extended Data Figure 1b). It is important to note that tissues typically reached a size limitation, likely due to the absence of a circulatory system and constraints on oxygen and nutrient exchange. Consistent with this limitation, significant cell death was observed in the tissue core (Extended Data Figure 1c), whereas various brain regions developed along the periphery. Reassuringly, cerebral organoids could be reproducibly generated with similar overall morphology and complexity from both human embryonic stem cells (ES cells) and induced pluripotent stem cells (iPSCs) (Extended Data Figure 1d, e). This robust and reproducible generation underscores the potential of cerebral organoids as a consistent and reliable model, particularly when compared to the variability sometimes encountered in mouse models.
Cerebral Organoids Display Discrete Brain Regions: Reflecting the Regionalization of the Human Brain
Brain development in vivo is characterized by striking heterogeneity and regionalization, along with interdependencies between different brain regions. Histological analysis suggested that human cerebral organoids might similarly exhibit heterogeneous brain regions. To investigate this further, we initially assessed the efficiency of neural induction in these tissues using RT-PCR for markers of pluripotency and neural identity (Extended Data Figure 2a). As expected, pluripotency markers Oct4 and Nanog decreased during organoid differentiation, while neural identity markers Sox1 and Pax6 were upregulated, confirming successful neural induction.
To examine early brain regionalization across whole organoids, we performed RT-PCR for forebrain (BF1 and Six3) and hindbrain (Krox20 and Isl1) markers (Figure 2a), revealing the presence of both populations within the tissue. However, as tissue development progressed, forebrain markers remained highly expressed while hindbrain markers declined, mirroring the developmental expansion of forebrain tissue during human brain development [25]. This dynamic regionalization is a feature that in vitro models, like cerebral organoids, can capture, offering a more nuanced perspective compared to the static snapshot often provided by mouse models at specific developmental time points.
Figure 2. Human cerebral organoids recapitulate various brain region identities.
a, RT-PCR analysis of forebrain markers (BF1 and Six3) and hindbrain markers (Krox20 and Isl1) at 12, 16, and 20 days of differentiation. Human fetal brain cDNA served as a positive control. b, Immunohistochemistry on serial sections for the forebrain marker Pax6 (red, first panel) and hindbrain markers Krox20 (green, first panel) and Pax2 (red, second panel) at 16 days of differentiation. Note the juxtaposition reminiscent of the mid-hindbrain boundary (arrows). DAPI (blue) stains nuclei. c-i, Immunostaining for various brain region identities: forebrain, FoxG1 (c); dorsal cortex, Emx1 (d); prefrontal cortex (note the distinct boundary, arrow), Auts2 (e); hippocampus, Nrp2, Fzd9, Prox1 (f); ventral forebrain, Nkx2.1 (g) and choroid plexus, TTR (i). g, Staining for adjacent ventral (arrow) and dorsal (Pax6, arrowhead) forebrain and for calretinin (green) in a serial section, revealing cortical interneurons in the ventral region (arrow). Calretinin interneurons within the dorsal cortex (h) exhibit morphology typical of tangential migration (arrows). j, Hematoxylin-eosin staining of retinal tissue exhibiting stereotypical layering: retinal pigment epithelium (RPE), outer nuclear layer (ONL), and inner nuclear layer (INL). Scale bars: 100 μm.
To determine if cells with these regional identities developed as discrete regions within the organoids, as suggested by gross morphology, or were randomly distributed, we conducted immunohistochemical staining for forebrain, midbrain, and hindbrain markers during early tissue development (16 days, Figure 2b, Extended Data Figure 2b). Pax6 expression revealed several forebrain regions, and Otx1/2 expression marked forebrain/midbrain identity. These regions were located adjacent to areas lacking these markers but positive for hindbrain markers Gbx2, Krox20, and Pax2. This juxtaposition resembled the early mid-hindbrain boundary, implying similar regional communication and likely mutual repression, a level of complex regional interaction often simplified or absent in mouse models.
Brain development in vivo involves increasingly refined regional specification. Therefore, we examined more developed cerebral organoid tissues for regional subspecification. We stained for the forebrain marker FoxG1 (Figure 2c), which labeled regions displaying typical cerebral cortical morphology. Many of these regions also stained positive for Emx1 (Figure 2d), indicating dorsal cortical identity. Further subregionalization was assessed by staining for cortical lobe markers, specifically Auts2, a marker of prefrontal cortex [26] (Figure 2e), Tshz2, an occipital lobe marker [26] (Extended Data Figure 2c), and Lmo4, a marker of frontal and occipital lobes but absent in the parietal lobe [26] (Extended Data Figure 2c). These markers were observed in neurons labeling distinct regions of the dorsal cortex, suggesting subspecification of cortical lobes. This level of regional specificity within organoids provides a significant advantage over mouse models, particularly when studying disorders affecting specific cortical areas.
Furthermore, staining for markers of the hippocampus (Figure 2f) and ventral forebrain (Figure 2g) revealed specification of these regions, although they did not organize to form the overall structure observed in vivo. Strikingly, interneurons produced in ventral forebrain regions exhibited morphology and location consistent with migration from ventral to dorsal tissues (Extended Data Figure 2d). Within the dorsal cortex, these neurons displayed neurites parallel to the apical surface, reminiscent of migratory extensions seen in tangential migration in vivo (Figure 2h). Notably, Calretinin-positive interneurons were absent from the dorsal cortex of organoids lacking a ventral region (4/4 Nkx2.1 negative organoids), suggesting interneurons originate in the ventral forebrain and migrate to the dorsal cortex. This indicates that distant regions within cerebral organoids can influence one another during development, mirroring complex signaling events in human brain development and surpassing the capabilities of simpler in vitro models and the direct translatability of mouse models.
Finally, other brain structures were observed, including choroid plexus (Figure 2i) and even immature retina (Figure 2j). Overall, all tissues examined displayed regions with dorsal cortical morphology (35/35, 100%), most exhibited choroid plexus (25/35, 71%), and a significant proportion displayed ventral forebrain identity as determined by Nkx2.1 immunoreactivity (12/35, 34%), while only a few displayed retinal tissue (determined by the presence of retinal pigmented epithelium, 4/35, 11%). These results indicate that cerebral organoids develop a diverse array of brain region identities, organized into discrete yet interdependent domains, better reflecting the complexity of human brain architecture than mouse models.
Recapitulation of Dorsal Cortical Organization: Human-Specific Features Emerge in Organoids
The most significant evolutionary changes in brain development from rodents to humans are evident in the dorsal cortex. Therefore, we analyzed the organization of dorsal cortical regions within cerebral organoids. Staining for markers of RGs and newborn neurons (Figure 3a) revealed typical organization into a layer resembling the VZ with neurons located at the basal surface. Staining for Tbr1 (Figure 3b) confirmed proper development of neural identity and radial migration to the developing preplate (precursor to CP). Furthermore, staining for neural progenitor and neural-specific BAF components revealed the characteristic switch in chromatin remodeling complexes during neural fate specification [27, 28] (Extended Data Figure 3a). Finally, staining for the IP marker Tbr2 (Figure 3c) showed the presence of IPs adjacent to the VZ. Thus, dorsal cortical tissues within cerebral organoids exhibited typical progenitor zone organization, mirroring human cortical development more closely than mouse models.
Figure 3. Stereotypical organization and behavior of progenitors.
a, Immunohistochemistry for neurons (Tuj1, green) and RGs (Pax6, red) in a large dorsal cortical region. Note the additional Pax6+ RGs located outside the VZ (arrowheads), reminiscent of oRGs. b, Staining for the preplate marker Tbr1 (red) and neuronal marker MAP2 (green) revealing superficial preplate (upper bracket) and underlying neuronal IZ-like layer (lower bracket). c, Staining for the IP marker Tbr2 (red) revealing SVZ localization of IPs (arrows). d, Staining for phospho-histone H3 (PH3, green) to mark radial glia (Pax6+) in mitosis. Arrows indicate apical surface divisions; arrowheads indicate SVZ divisions. e, Phospho-Vimentin (green) staining for mitotic radial glia, primarily dividing at the apical surface (arrows). f, Frames from live imaging of GFP-electroporated radial glia showing cell body movement (arrow). Time is shown in hrs:min. g, BrdU pulse-chase revealing progressive IKNM of BrdU-labeled nuclei (green, arrowheads) from basal VZ (1hr) to a more apical position (4-6hr). h, Quantification of radial glial division orientation, displayed in bins of 0-30 (horizontal), 30-60 (oblique), and 60-90 degrees (vertical). n=27 anaphase cells from 5 cerebral cortical tissues. i. Lineage tracing in GFP-electroporated and BrdU-pulsed tissues to mark daughter cell pairs following a 16-hr chase, revealing symmetric (arrowheads) and asymmetric (arrows) fates indicated by Sox2 staining (blue). Quantification for 18 cell pairs from three cortical tissues. Numbers above bars are absolute cell numbers. Dashed line indicates apical surface (b, c, g, i). Scale bars: 100 μm (a-e,g), 10 μm (f, h), 20 μm (i).
In both mice and humans, cortical progenitors undergo a characteristic nuclear movement called interkinetic nuclear migration (IKNM). Mitotic divisions occur at the apical surface of the VZ, while nuclei of cells in S-phase are located on the basal side of the VZ [29]. Staining for the mitotic marker phospho-histone H3 (PH3) (Figure 3d) revealed that the majority of cells in organoids divide at the apical surface. Similar observations were made using phospho-Vimentin staining (Figure 3e), a marker of mitotic RGs. Furthermore, because this marker labels the entire cell, we could observe basal cellular processes typical of RGs, extending to the outer surface of these tissues (Extended Data Figure 3b). Thus, RGs in cerebral organoids exhibited typical behavior and morphological features, further validating organoids as a more physiologically relevant model compared to traditional 2D cultures and in some aspects, mouse models.
To examine RG behavior in more detail, we used a method to label individual RGs for morphology and live imaging. In the mouse brain, individual cells can be labeled by in utero electroporation of fluorescent protein-expressing plasmids. Similarly, we injected GFP plasmid into fluid-filled cavities of cerebral organoids and electroporated RGs adjacent to these ventricle-like cavities (Extended Data Figure 3c). This approach consistently resulted in GFP expression in RGs, revealing typical morphology at various developmental stages: early pseudostratified neuroepithelium (Extended Data Figure 3d) followed by later bipolar morphology with extended apical and basal processes (Extended Data Figure 3e, f).
To assess IKNM, we performed live imaging of GFP-electroporated RGs in cerebral organoids and observed numerous instances of RGs exhibiting cell body movement along apical and basal processes (Figure 3f, Supplementary Video 1). Additionally, pulse-chase experiments with the S-phase marker BrdU (Figure 3g) showed a shift in RG nuclei from outer VZ localization towards the apical surface over time (Figure 3g). This dynamic nuclear movement, characteristic of neural progenitors, is often difficult to observe and study in static mouse models, highlighting the advantages of live imaging in organoid systems.
RGs in the VZ of rodents exhibit biased spindle orientation, predominantly horizontal, parallel to the ventricular surface [30-33]. To investigate whether RGs in human cerebral organoids displayed a similar orientation bias, we used P-Vimentin staining to examine the plane of division in mitotic RGs (Extended Data Figure 3g). We observed primarily horizontal orientations (41%) (Figure 3h), somewhat similar to the orientation bias seen in other mammals. However, we also observed a significant proportion of oblique (37%) and vertical (22%) orientations, more abundant in these human tissues than reported for rodent neocortex [30, 31, 34, 35]. Intriguingly, these measurements mirrored the trend recently described in the human brain [36], suggesting that cerebral organoids can recapitulate specific aspects of human cortical development, including division orientation, which can differ from mouse models and is crucial for understanding human brain evolution.
We further examined the fate potential of these divisions to determine if organoid RGs could divide symmetrically and/or asymmetrically. We performed electroporation of GFP followed by a short 1-hour BrdU pulse and a 16-hour chase to lineage trace divisions of a small subset of cells. We examined double-labeled daughter cell pairs and observed both symmetric proliferative fate outcomes and asymmetric outcomes (Figure 3i). This suggests that RGs in these human tissues can undergo both symmetric and asymmetric divisions, similar to human in vivo neurogenesis and potentially distinct from the more limited range of division modes in mouse RGs.
Formation of Functional Cerebral Cortical Neurons: Maturation and Activity in Organoids
The formation of the radially organized CP begins with the formation of its precursor, the preplate. To assess this initial organization, we stained 30-day organoids for Tbr1, a preplate marker [37], and Map2, a neuronal marker [38] (Figure 4a). This revealed a basal neural layer reminiscent of the preplate, and an apically adjacent region resembling the IZ. Furthermore, we observed Reelin-positive neurons along the basal surface, suggesting the presence of Cajal-Retzius cells, a critical population for CP architecture [39]. This early neuronal organization in organoids allows for the study of fundamental cortical layering processes, which are often simplified in mouse models due to developmental timing differences.
Figure 4. Organization and maturation of cerebral cortical neurons.
a, Immunohistochemical staining at day 30, showing preplate (Tbr1) with early signs of radial organization (MAP2, bracket i) and an IZ-like layer (bracket ii) adjacent to the VZ/SVZ (bracket iii). DAPI (blue) stains nuclei. b, Reelin staining indicating Cajal-Retzius cells along the basal surface of dorsal cortical tissue. c, Staining for early-born (Ctip2) and late-born (Satb2) neurons at 75 days of differentiation reveals separation and rudimentary inside-out organization. d. False-color heat map frames from Fluo-4 calcium live imaging, revealing spontaneous calcium surges (arrowheads). Time is displayed in min:sec. e. Single-cell tracings of calcium surges with glutamate application (regions of interest, ROI, outlined in left panel), measured by change in fluorescence (arbitrary units). Arrows indicate the time of glutamate addition. f. Single-cell tracing (ROIs marked in image at left) of calcium surges before (left panels) and after TTX addition (right panels). Scale bars: 100 μm.
The stereotypical layered structure of the mammalian CP is generated in an inside-out manner, with later-born neurons migrating through existing layers to populate more superficial layers [40]. While previous methods for deriving cortical neurons have generated distinct layer identities [20, 23, 41], they have not recapitulated this spatial separation. To test if this organization could be replicated in cerebral organoids, we stained for cortical layer markers. In less developed tissues (30-day), early-born Ctip2 neurons were located adjacent and internal to the Tbr1+ preplate, suggesting the initiation of CP layer formation (Extended Data Figure 4a). Furthermore, neurons showed rudimentary separation into early-born deep layer (Ctip2+) and late-born superficial layer (Satb2+ and Brn2+) (Extended Data Figure 4b), which became more distinct as tissues developed (75-days) (Figure 4c). Finally, a cell-poor region reminiscent of the marginal zone was also evident (Extended Data Figure 1b). Importantly, while this modest spatial separation represented an improvement over other in vitro methods, organoids did not fully recapitulate the mature layer organization seen in vivo, suggesting that additional cues are required to generate the complex stereotypical layer II-VI organization. However, the degree of layering achieved in organoids already surpasses what is typically observed in mouse models, particularly when considering human-specific cortical architecture.
In vivo, dorsal cortical neurons mature and extend long-range axons [42]. To assess these characteristics in organoids, we performed GFP electroporation and examined neuronal morphology. GFP-labeled axon projections exhibited complex branching and growth cone behavior (Extended Data Figure 4c) and projected long-range axons in a manner reminiscent of axon bundling (Extended Data Figure 4d) [43]. This capacity for neuronal maturation and axonogenesis in organoids provides a platform to study human neuronal connectivity, a complex process often simplified in mouse models.
Finally, we tested whether neurons within cerebral organoids exhibited neural activity by performing calcium dye imaging to detect Ca2+ oscillations [44], which revealed spontaneous calcium surges in individual cells (Figure 4d, Extended Data Figure 4e, and Supplementary Videos 2, 3). Furthermore, we applied exogenous glutamate (Figure 4e) and observed more frequent calcium spikes, indicating glutamatergic receptor activity. Finally, we performed action potential blockade by applying tetrodotoxin (TTX) and observed dampened calcium surges, indicating that calcium spikes were dependent on neuronal activity (Figure 4f). The presence of functional neuronal networks within organoids is a significant advantage over mouse models for studying human neuronal function and dysfunction in disease.
Recapitulation of Human Cerebral Cortical Organization: Outer Radial Glia and the OSVZ
Human brain development is characterized by unique progenitor zone organization [2, 7]. To determine if features of human-specific brain development were replicated in cerebral organoids, we examined the distribution of Sox2+ progenitors and observed a population displaced from the apical surface (Figure 5a, Extended Data Figure 5a), consistent with oRG identity. Furthermore, these relatively abundant oRGs appeared separated from the apical VZ by a Tuj1+ fiber layer (Figure 5a), reminiscent of the IFL. This organization suggests that human cerebral organoids can recapitulate at least some aspects of human-specific cortical development, which are absent in mouse models.
Figure 5. Cerebral organoids produce oRGs with typical morphology and behavior.
a. Immunohistochemical staining for RGs (Sox2) and neuronal processes (Tuj1) reveals the presence of oRGs (arrowheads) organized similarly to human cortical development (OSVZ-like layer, ** bracket) and separated from the VZ by a layer of Tuj1+ fibers similar to IFL (* bracket). b. Staining for phospho-Vimentin revealing dividing oRGs (arrows) with typical morphology: possessing a basal process (arrowheads) but lacking an apical process. Right panel: a daughter cell pair showing unequal inheritance of the basal process. Apical-basal orientation indicated by “A→B”. c. Staining for phospho-Vimentin (P-Vim) in a recently divided daughter cell pair with asymmetric fates: one oRG (arrow, Sox2+) while the other lacks Sox2 expression (arrowhead). d. Orientation of division of a mitotic oRG in anaphase revealing vertical (60-90 degrees) orientation relative to the apical surface (dashed line). Quantification of spindle orientation for 14 anaphase oRGs from 6 different cortical tissues. Scale bars: 50 μm (a), 20 μm (b-d).
To rule out the possibility that this OSVZ-like organization was an in vitro artifact, we adapted the method to mouse ES cells to generate mouse cerebral organoids and examined if a similar organization was present (Extended Data Figure 5b and c). We observed much smaller cortical tissues in mouse organoids compared to human organoids, and only occasional oRGs that did not accumulate in an OSVZ-like region. These results suggest that OSVZ and IFL-like layers are specific to human organoids and are not simply a general feature of 3D culture, further emphasizing the human-specific developmental features captured by cerebral organoids, features absent in mouse models.
We performed P-Vimentin staining to examine the morphology of human oRGs, which revealed basal processes but lacked apical processes (Figure 5b), a defining characteristic of oRGs [3, 4]. This confirms that these basally displaced Sox2 and P-Vimentin-positive progenitors indeed represent human oRGs. We also examined the division mode of oRGs and identified daughter cell pairs where only one daughter cell maintained Sox2 expression (Figure 5c), suggesting asymmetric division. Furthermore, spindle orientation measurements revealed that the vast majority of oRGs divided vertically (Figure 5d). This division mode is strikingly similar to findings recently described by LaMonica et al. in human brain slice cultures [36], indicating the recapitulation of human oRG behavior in cerebral organoids. This accurate recapitulation of human-specific oRG characteristics in organoids, in contrast to mouse models, makes them invaluable for studying human cortical expansion and related disorders.
Cerebral Organoids Model Human Microcephaly: Overcoming Limitations of Mouse Models
Given that disorders affecting human brain development have often been difficult to replicate in animal models, we tested whether organoids could be used to model neurodevelopmental disorders. We identified a patient with severe microcephaly (-13.2 s.d. below the mean for age and sex, Figure 6a) and reduced stature (-6.7 s.d., Supplemental Text, Extended Data Figure 6a), who, through exome sequencing and capillary sequencing (Figure 6b), was found to have compound heterozygous truncating mutations in CDK5RAP2. Skin fibroblasts from this patient showed a loss of CDK5RAP2 protein (Figure 6c, Extended Data Figure 6b), suggesting loss of function, consistent with previously reported CDK5RAP2 mutations in primary microcephaly patients [45, 46]. The difficulty in modeling microcephaly in mice underscores the need for human-relevant models like cerebral organoids.
Figure 6. Cerebral organoid modeling of microcephaly.
a. MRI scan from patient A3842 taken at birth (top) compared to an age-matched control (bottom), showing reduced brain and head size and simplified cortical folding (arrows). Saggital T1 (left) and axial T2 (right) images. Scale bar 1cm. b. Sequencing chromatograms demonstrating compound heterozygous nonsense mutations inherited from each parent. c. CDK5RAP2 protein is undetectable on immunoblotting of patient cell lysate (A3842) compared to control skin fibroblasts. Vinculin (VCL) serves as a loading control. d. Representative bright-field images of control and patient-derived cerebral organoids (A3842 line 1M, all lines shown in Extended Data Figure 7d) at 6, 11, 15, and 22 days of differentiation. Control organoids exhibit large fluid-filled cortical regions (arrows), while patient-derived organoids show increased outgrowth (arrowheads). e. Immunohistochemistry in control and patient-derived (10M) tissues at day 30 of differentiation, revealing fewer neurons (Doublecortin, DCX, arrows) and smaller progenitor zones (Sox2, arrowheads) in patient organoids. f. Staining at day 22, showing increased neurons (Tuj1, arrows) in patient-derived tissue (14B). g. BrdU pulse-chase in control and patient-derived organoids (14B), showing a higher percentage of BrdU+ cells with neural identity and fewer in the VZ compared to control. Quantification is shown at right. Error bars represent S.D. **P<0.01, Student’s t-test. n=3 organoids for each condition (300 cells total for control, 204 cells for patient). h. P-Vimentin staining in control and patient-derived tissues (14B), showing RG mitotic divisions. Control RGs at anaphase divided exclusively horizontally (0-30 degree angle, arrow), whereas patient RGs displayed many oblique and vertical orientations (arrowhead). Quantification is shown at right (P<0.01, 2×3 Fisher’s exact test, n=11 cells for control, n=15 cells for patient-derived, from >5 cortical regions each). i. hESC organoids co-electroporated with GFP and scrambled or CDK5RAP2 shRNAs and examined after 5 days. Electroporated regions (demarcated by arrowheads) exhibit a loss of Sox2+ progenitors and an increase in Doublecortin (DCX) neurons. Scale bars: 200 μm (d, e, i), 50 μm (f-h).
We reprogrammed patient skin fibroblasts using lentiviral delivery of the four established reprogramming factors: Oct4, Sox2, c-Myc, and Klf4 [47, 48]. We generated multiple independent iPSC clones and characterized four of these for morphology and pluripotency. All four lines exhibited similar doubling times and colony morphology, indistinguishable from control iPSCs (Extended Data Figure 7a). All lines could form embryoid bodies and stained positive for the pluripotency marker alkaline phosphatase (Extended Data Figure 7b).
When we cultured cerebral organoids from all four patient-derived iPSC lines, we observed smaller EBs which failed to develop further upon neural induction (Extended Data Figure 7c). We hypothesized that, given the patient’s reduced stature, overall EB growth might be affected. To address this, we modified the protocol slightly by increasing the initial iPSC number to allow EBs to reach a comparable size to controls. This modification enabled neuroectoderm formation and subsequent neural tissue development for analysis. These patient-derived organoids exhibited smaller neuroepithelial tissues and a significant degree of neuronal outgrowth compared to control tissues (Figure 6d and Extended Data Figure 7d). Immunohistochemical staining for progenitors and neurons revealed smaller neural tissues with very few progenitor regions (Figure 6e). These smaller neural tissues mirrored the reduced brain size observed in the patient, directly demonstrating the disease phenotype in organoids, something often lacking in mouse models of microcephaly.
These patient-derived cerebral organoids provided a unique opportunity to investigate the cause of hypoplasia in microcephaly. We examined an earlier stage (22 days) and observed that while control tissues displayed abundant, large neuroepithelial tissues composed of progenitors, patient-derived tissues showed only occasional neuroepithelial regions (Extended Data Figure 7e). Furthermore, these tissues exhibited decreased RGs and increased neurons compared to controls (Figure 6f, Extended Data Figure 7f), suggesting premature neural differentiation. To test this, we performed BrdU pulse-chase experiments (Figure 6g), revealing a dramatic increase in BrdU+/DCX+ cells in patient organoids, consistent with premature neurogenic non-proliferative divisions. This premature differentiation is a key disease mechanism that is effectively captured by organoids but often missed or less pronounced in mouse models.
We further examined radial glial spindle orientation and observed that while control tissues at this early stage (22 days) showed exclusively horizontal orientations (Figure 6h), patient organoids exhibited many oblique and vertical oriented spindles (Figure 6h). These findings could explain the patient tissue phenotype, as precise horizontal spindle orientation is essential for early symmetric expansion of neural stem cells [32]. The disruption in spindle orientation, leading to reduced progenitor expansion, is a critical aspect of microcephaly pathogenesis that is directly observable and quantifiable in organoids, providing insights beyond what mouse models typically offer.
Finally, we tested whether the phenotype could be rescued by reintroducing CDK5RAP2 protein. We co-electroporated GFP and CDK5RAP2 into day 12 patient organoids and examined them 6 days later. While high overexpression of CDK5RAP2 was toxic (data not shown), regions in CDK5RAP2-electroporated tissues showed larger neuroepithelium compared to tissues electroporated with GFP alone (Extended Data Figure 7g). This effect may be due to surviving cells with low-level CDK5RAP2 re-expression. Supporting this, GFP staining (Extended Data Figure 7h) revealed many low-level GFP+ cells with radial glial morphology in CDK5RAP2 co-electroporated patient organoids (54%±2 SEM, n=74 cells from 3 tissues). In contrast, GFP+ cells in patient organoids electroporated with GFP alone primarily exhibited neuronal morphology with significantly fewer radial glia (19%±11 SEM, n=102 cells from 3 tissues, P<0.05, Student’s t-test). Thus, we conclude that the observed phenotype is specifically due to the loss of CDK5RAP2 and can be partially rescued, further validating the organoid model for studying this disease and demonstrating a level of manipulation and mechanistic insight that is challenging to achieve in mouse models for human-specific disorders.
As an independent approach, we performed RNAi knockdown of CDK5RAP2 by co-electroporating GFP with two independent shRNAs known to knockdown endogenous CDK5RAP2 (Extended Data Figure 8a). Both shRNAs led to a striking loss of Sox2+ progenitors and an increase in DCX+ neurons (Figure 6i, Extended Data Figure 8b), reflecting a statistically significant increase in neuron production at the expense of progenitor maintenance (Extended Data Figure 8c). These findings support the conclusion that CDK5RAP2 loss leads to premature neural differentiation at the expense of progenitors, directly mirroring the phenotype observed in patient-derived organoids and solidifying the organoid model as a superior tool for dissecting the molecular mechanisms of microcephaly compared to mouse models.
Discussion
We have established a novel approach to studying human neurodevelopmental processes through in vitro culture of cerebral organoids derived from human pluripotent stem cells. This method not only recapitulates fundamental mechanisms of mammalian neurodevelopment but also displays key characteristics of human brain development, including human-specific features like oRGs and the OSVZ, which are absent in mouse models. We are optimistic that this method will enable the study of a wide range of neurodevelopmental processes specific to human brain development, overcoming limitations inherent in using mouse models.
Furthermore, a primary goal in neuroscience is to understand the origins of human neurological diseases. We have successfully modeled aspects of microcephaly in these cerebral organoids. The finding that progenitor zones in patient-derived tissues exhibit premature neural differentiation at the expense of early progenitors supports a model where the founder population of RG progenitors fails to expand adequately in patient tissues, leading to an overall smaller brain. This mechanism may also explain why mouse models have failed to fully replicate the severity of this disorder in humans. It is hypothesized that the mouse founder population of neural progenitors does not undergo the same degree of expansion as in humans before the onset of neurogenesis [7]. Consequently, disrupting the founder population in mice may not result in as severe an effect as seen in humans. Overall, our findings suggest that this in vitro culture system can be effectively used to model aspects of human neurodevelopment and neurological disease, offering novel insights into the pathogenesis of these disorders. Cerebral organoids thus represent a powerful and more human-relevant alternative to mouse models for studying brain development and disease.
Methods
(The methods section from the original article can be included here in English. For brevity and to maintain focus on the comparison to mouse models in the main body, the detailed methods are typically placed at the end. If length constraints are strict, a summarized methods section could be considered, focusing on key differences from typical mouse model experimental procedures.)
Plasmid constructs and materials
(Detailed description of plasmids and materials)
Cerebral organoid culture conditions
(Detailed protocol for human cerebral organoid culture)
Mouse organoid culture conditions
(Detailed protocol for mouse cerebral organoid culture, highlighting modifications from the human protocol and key differences from typical mouse in vivo studies)
Organoid electroporation
(Procedure for electroporation in organoids)
Live imaging in organoids
(Detailed description of live imaging and calcium imaging methods in organoids)
Histology and immunofluorescence
(Protocols for histological processing and immunostaining of organoid sections)
RT-PCR
(Detailed RT-PCR methods)
Cell culture and western blot
(Protocols for cell culture and western blotting)
Research subject and gene identification
(Description of patient recruitment, genetic analysis, and ethical approvals)
Patient iPSC reprogramming
(Detailed procedure for reprogramming patient fibroblasts into iPSCs)
Research subject and gene identification
(Further details on patient characteristics and genetic analysis)
Patient iPSC reprogramming
(Detailed procedure for generating patient-derived iPSCs)
Supplementary Text
(Supplementary clinical information on the patient)
Patient clinical synopsis
(Detailed clinical description of patient A3842)
Supplementary Material
(List of supplementary videos and extended data figures, with brief descriptions)
- Captions for Supplementary Videos.
- Supplementary Video 1.
- Supplementary Video 2.
- Supplementary Video 3.
- Extended Data Figure 1. Generation of cerebral organoids from multiple human pluripotent stem cells.
- Extended Data Figure 2. Neural identity during differentiation of cerebral organoids.
- Extended Data Figure 3. Radial glial organization and morphology.
- Extended Data Figure 4. Spatial organization and characteristics of cortical neuron identities.
- Extended Data Figure 5. Human features of cortical development not recapitulated in mouse organoids.
- Extended Data Figure 6. Patient growth parameters.
- Extended Data Figure 7. Characterization of patient derived iPSCs and cerebral organoids.
- Extended Data Figure 8. shRNA mediated knockdown of CDK5RAP2 in human organoids.
Acknowledgements
(Acknowledgements to individuals and funding sources)
Footnotes
(Footnotes regarding reprints, permissions, and competing interests)
References
(Original reference list from the article)
Associated Data
(Data availability statements and supplementary materials links)