At COMPARE.EDU.VN, we understand the intricacies of chemical reactions and stoichiometry. Mole-to-mole ratios are fundamental in understanding chemical reactions, and while they are most directly applicable at equilibrium, they also play a crucial role in understanding reactions that are not at equilibrium. This article explores how mole ratios relate to equilibrium and how they can be applied in various chemical scenarios, enhancing your understanding of chemical transformations.
1. What Is a Mole Ratio and Why Is It Important?
A mole ratio is a conversion factor derived from the coefficients of a balanced chemical equation. These coefficients represent the relative number of moles of each reactant and product involved in the reaction. Mole ratios are essential for stoichiometric calculations, allowing us to predict the amount of product formed from a given amount of reactant or vice versa.
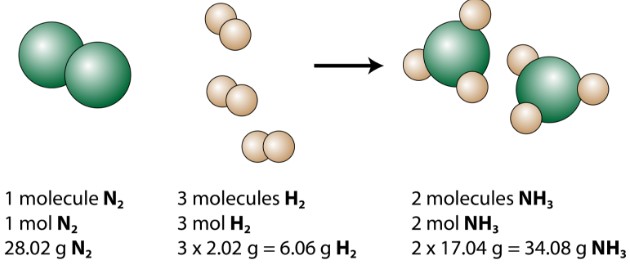
For example, consider the balanced chemical equation for the synthesis of ammonia:
[N_2(g) + 3H_2(g) rightleftharpoons 2NH_3(g)]
This equation tells us that one mole of nitrogen reacts with three moles of hydrogen to produce two moles of ammonia. Therefore, we can derive the following mole ratios:
- 1 mol N₂ : 3 mol H₂
- 1 mol N₂ : 2 mol NH₃
- 3 mol H₂ : 2 mol NH₃
These ratios are crucial for determining the amounts of reactants needed or products formed in a chemical reaction.
2. The Role of Equilibrium in Chemical Reactions
Chemical equilibrium is a state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products. Equilibrium is described by the equilibrium constant (K), which provides insight into the extent to which a reaction will proceed to completion.
2.1. Understanding Equilibrium Constant (K)
The equilibrium constant (K) is a ratio of the concentrations of products to reactants at equilibrium, each raised to the power of their stoichiometric coefficients. For the general reaction:
[aA + bB rightleftharpoons cC + dD]
The equilibrium constant expression is:
[K = frac{[C]^c [D]^d}{[A]^a [B]^b}]
Where [A], [B], [C], and [D] are the equilibrium concentrations of the reactants and products, and a, b, c, and d are their respective stoichiometric coefficients.
2.2. How Equilibrium Affects Mole Ratios
At equilibrium, the mole ratios derived from the balanced chemical equation help define the relative amounts of reactants and products present. The equilibrium constant (K) determines the extent to which the reaction proceeds towards product formation. A large K indicates that the reaction favors the formation of products, while a small K indicates that the reaction favors the reactants.
3. Can Mole Ratios Be Used at Non-Equilibrium Conditions?
Yes, mole ratios can be used at non-equilibrium conditions, but with careful consideration. While the direct application of mole ratios is most straightforward at equilibrium, they are still valuable in understanding and predicting reaction outcomes under non-equilibrium conditions.
3.1. Initial Stoichiometry and Limiting Reactants
Before a reaction reaches equilibrium, the mole ratios are used to determine the limiting reactant—the reactant that is completely consumed first, thus dictating the maximum amount of product that can be formed. The initial stoichiometry of the reaction, based on mole ratios, helps in predicting the theoretical yield of the products.
For example, if you start with 2 moles of N₂ and 4 moles of H₂, according to the balanced equation, N₂ is the limiting reactant because it requires 3 moles of H₂ for every mole of N₂. Thus, only (frac{4}{3}) moles of N₂ can react, limiting the amount of NH₃ produced.
3.2. Reaction Progress and Extent of Reaction
The extent of reaction, often denoted by the variable ‘x,’ quantifies how far a reaction has progressed towards equilibrium. By using an ICE (Initial, Change, Equilibrium) table and mole ratios, one can track the changes in concentrations of reactants and products as the reaction proceeds.
Consider the ammonia synthesis again:
| N₂ | 3H₂ | 2NH₃ | |
|---|---|---|---|
| Initial (I) | 2 mol | 4 mol | 0 mol |
| Change (C) | -x | -3x | +2x |
| Equilibrium (E) | 2-x | 4-3x | 2x |

Here, ‘x’ represents the moles of N₂ that have reacted. The mole ratios dictate the changes in the amounts of H₂ and NH₃. This approach helps estimate concentrations at any point during the reaction, not just at equilibrium.
3.3. Using Mole Ratios in Kinetic Studies
In chemical kinetics, mole ratios are crucial for understanding reaction mechanisms and rate laws. The rate of a reaction often depends on the concentrations of reactants, and the changes in these concentrations are dictated by the mole ratios in the balanced equation.
For instance, if the rate law for the ammonia synthesis is given by:
[Rate = k[N_2][H_2]^2]
The rate at which N₂ and H₂ are consumed and NH₃ is formed is governed by their respective mole ratios. The rate of disappearance of H₂ is three times the rate of disappearance of N₂, and the rate of appearance of NH₃ is twice the rate of disappearance of N₂.
4. Practical Applications and Examples
To illustrate how mole ratios are used both at and away from equilibrium, let’s explore several practical examples.
4.1. Calculating Theoretical Yield at Non-Equilibrium
Suppose we react 5.0 moles of methane ((CH_4)) with 8.0 moles of oxygen ((O_2)) to produce carbon dioxide ((CO_2)) and water ((H_2O)). The balanced equation is:
[CH_4(g) + 2O_2(g) rightarrow CO_2(g) + 2H_2O(g)]
To find the limiting reactant:
- For (CH_4): 5.0 moles (CH_4) would require (5.0 times 2 = 10.0) moles (O_2)
- Since we only have 8.0 moles (O_2), oxygen is the limiting reactant.
Using the mole ratio of (O_2) to (CO_2) (2:1), the theoretical yield of (CO_2) is:
[8.0 : text{moles} : O_2 times frac{1 : text{mole} : CO_2}{2 : text{moles} : O_2} = 4.0 : text{moles} : CO_2]
4.2. Determining Equilibrium Concentrations
Consider the reaction:
[H_2(g) + I_2(g) rightleftharpoons 2HI(g)]
Initially, 1.0 mole of (H_2) and 2.0 moles of (I_2) are placed in a 1.0 L container. At equilibrium, the concentration of (HI) is 1.56 M. Calculate the equilibrium constant K.
First, set up the ICE table:
| (H_2) | (I_2) | 2(HI) | |
|---|---|---|---|
| Initial (I) | 1.0 M | 2.0 M | 0 M |
| Change (C) | -x | -x | +2x |
| Equilibrium (E) | 1.0-x | 2.0-x | 2x |
Since ([HI]) at equilibrium is 1.56 M:
[2x = 1.56]
[x = 0.78]
Now, calculate the equilibrium concentrations:
- ([H_2] = 1.0 – 0.78 = 0.22 : M)
- ([I_2] = 2.0 – 0.78 = 1.22 : M)
- ([HI] = 1.56 : M)
Finally, calculate K:
[K = frac{[HI]^2}{[H_2][I_2]} = frac{(1.56)^2}{(0.22)(1.22)} = frac{2.4336}{0.2684} approx 9.07]
4.3. Industrial Applications: Haber-Bosch Process
The Haber-Bosch process, crucial for ammonia production, exemplifies the use of mole ratios in industrial chemistry. The reaction:
[N_2(g) + 3H_2(g) rightleftharpoons 2NH_3(g)]
is optimized under high pressure and temperature to shift the equilibrium towards ammonia formation. Stoichiometric ratios ensure efficient use of reactants.
Engineers use mole ratios to determine the required amounts of nitrogen and hydrogen, maximizing ammonia yield while minimizing waste. The process continuously monitors and adjusts reactant flow to maintain optimal conditions for equilibrium, enhancing overall efficiency.
5. Common Pitfalls and How to Avoid Them
When working with mole ratios, it is crucial to avoid common mistakes that can lead to incorrect calculations and conclusions.
5.1. Forgetting to Balance the Chemical Equation
The most fundamental mistake is using an unbalanced chemical equation. The coefficients in the balanced equation are the basis for mole ratios. Always double-check that your equation is balanced before performing any stoichiometric calculations.
5.2. Incorrectly Identifying the Limiting Reactant
Incorrectly identifying the limiting reactant can lead to overestimation of the product yield. Ensure you correctly determine which reactant will be completely consumed first by comparing the mole ratios of reactants to their initial amounts.
5.3. Neglecting the Reaction Quotient (Q)
The reaction quotient (Q) is a measure of the relative amounts of products and reactants present in a reaction at any given time. Comparing Q to K can indicate the direction in which the reaction must shift to reach equilibrium. Neglecting Q can lead to incorrect predictions about the reaction’s progress.
5.4. Ignoring the Effects of Temperature and Pressure
Temperature and pressure can significantly affect the equilibrium position, especially for gaseous reactions. Le Chatelier’s principle states that a system at equilibrium will adjust to counteract any applied stress. Changes in temperature and pressure can alter the equilibrium constant and shift the reaction accordingly.
6. Advanced Techniques and Considerations
For more complex chemical systems, additional techniques and considerations may be necessary for accurate analysis.
6.1. Using Activity Instead of Concentration
In highly concentrated solutions, the effective concentration, or activity, of a species may differ significantly from its actual concentration. Using activities in equilibrium calculations provides more accurate results, especially for ionic species.
6.2. Dealing with Complex Equilibria
Some chemical systems involve multiple equilibria occurring simultaneously. These systems require solving multiple equilibrium expressions to determine the concentrations of all species accurately.
6.3. Applying Computational Chemistry
Computational chemistry methods can be used to predict equilibrium constants and reaction rates for complex reactions. These methods provide valuable insights into reaction mechanisms and can help optimize reaction conditions.
7. Real-World Applications of Mole Ratios
Mole ratios and stoichiometry are not just theoretical concepts; they are foundational to many real-world applications in various industries.
7.1. Pharmaceuticals
In the pharmaceutical industry, precise stoichiometry is crucial for synthesizing drug compounds. Ensuring the correct mole ratios of reactants is essential for maximizing yield and purity of the final product. Accurate calculations prevent the formation of unwanted byproducts and ensure the drug meets strict quality control standards.
For example, synthesizing aspirin involves reacting salicylic acid with acetic anhydride. The mole ratio between these reactants must be carefully controlled to achieve the desired product and minimize waste.
7.2. Agriculture
In agriculture, understanding mole ratios is important for formulating fertilizers and pesticides. Farmers and agricultural scientists use stoichiometry to determine the optimal amounts of nutrients needed for plant growth and the correct concentration of pesticides to control pests without harming the environment.
For example, nitrogen, phosphorus, and potassium (NPK) ratios in fertilizers are carefully balanced to meet the specific needs of different crops. Mole ratios help in calculating the mass of each component required to achieve the desired NPK ratio.
7.3. Environmental Science
Environmental scientists use mole ratios to monitor and control pollution. Stoichiometry helps in understanding chemical reactions involved in air and water pollution and in developing methods for remediation.
For example, flue gas desulfurization (FGD) involves reacting sulfur dioxide with calcium carbonate to remove it from industrial emissions. The mole ratio between these compounds is crucial for the efficient removal of sulfur dioxide and preventing acid rain.
7.4. Materials Science
In materials science, mole ratios are essential for designing and synthesizing new materials with specific properties. Stoichiometry helps in controlling the composition of materials and predicting their behavior under different conditions.
For example, in the synthesis of ceramics, the mole ratios of different metal oxides determine the final structure and properties of the ceramic material. Precise control of stoichiometry is crucial for achieving desired characteristics such as hardness, thermal stability, and electrical conductivity.
8. Conclusion: Mastering Mole Ratios for Chemical Success
In summary, mole ratios are indispensable tools in chemistry, applicable both at and away from equilibrium. At equilibrium, they define the relative amounts of reactants and products, governed by the equilibrium constant K. Away from equilibrium, they help determine limiting reactants, track reaction progress, and understand reaction kinetics.
Mastering mole ratios requires a solid understanding of balanced chemical equations, stoichiometry, and equilibrium concepts. By avoiding common pitfalls and employing advanced techniques, you can accurately predict and control chemical reactions for a wide range of applications.
8.1. Need More Help with Comparisons?
Are you struggling to compare complex chemical processes or products? Do you need help understanding how different variables affect reaction outcomes? Visit COMPARE.EDU.VN for comprehensive comparisons, detailed analyses, and expert insights. Whether you’re a student, researcher, or industry professional, our resources can help you make informed decisions and achieve success in your chemical endeavors.
At COMPARE.EDU.VN, we provide objective comparisons to help you make informed decisions. We understand that comparing different products, services, and ideas can be overwhelming, so we provide easy-to-understand comparisons, highlighting the pros and cons of each option. Whether you’re choosing a new chemical process, deciding on the right fertilizer, or selecting materials for a new product, COMPARE.EDU.VN can help you make the right choice. Contact us today at 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090.
9. FAQ: Frequently Asked Questions About Mole Ratios
9.1. What is the difference between mole ratio and stoichiometric ratio?
A mole ratio is derived from the coefficients of a balanced chemical equation and represents the ratio of moles of any two substances in the reaction. A stoichiometric ratio refers to the general quantitative relationship between reactants and products in a chemical reaction, often encompassing mole ratios but also including mass ratios and volume ratios.
9.2. How do you calculate mole ratios from a chemical equation?
To calculate mole ratios, simply use the coefficients from the balanced chemical equation. For example, in the reaction (2H_2 + O_2 rightarrow 2H_2O), the mole ratio of (H_2) to (O_2) is 2:1, and the mole ratio of (H_2O) to (O_2) is 2:1.
9.3. Can mole ratios be used for reactions that do not go to completion?
Yes, mole ratios can be used for reactions that do not go to completion, but it is important to consider the equilibrium constant (K) and the extent of reaction. Mole ratios help in determining the amounts of reactants consumed and products formed, even if the reaction does not reach 100% completion.
9.4. What is the significance of the limiting reactant in mole ratio calculations?
The limiting reactant is the reactant that is completely consumed first in a chemical reaction. It determines the maximum amount of product that can be formed. Identifying the limiting reactant is crucial for accurate mole ratio calculations and for predicting the theoretical yield of the reaction.
9.5. How does temperature affect mole ratios in a chemical reaction?
Temperature can affect the equilibrium position of a chemical reaction, especially if the reaction is endothermic or exothermic. According to Le Chatelier’s principle, increasing the temperature will shift the equilibrium towards the side that absorbs heat (endothermic), while decreasing the temperature will shift the equilibrium towards the side that releases heat (exothermic).
9.6. Are mole ratios applicable to gas-phase reactions?
Yes, mole ratios are applicable to gas-phase reactions. In gas-phase reactions, the ideal gas law ((PV = nRT)) can be used to relate the number of moles to the volume, pressure, and temperature of the gases. Mole ratios are essential for stoichiometric calculations involving gas volumes.
9.7. How do you use an ICE table with mole ratios to solve equilibrium problems?
An ICE (Initial, Change, Equilibrium) table is used to organize and calculate the equilibrium concentrations of reactants and products. The initial concentrations, changes in concentration based on mole ratios, and equilibrium concentrations are listed in the table. Using the equilibrium concentrations, the equilibrium constant (K) can be calculated.
9.8. What are the common mistakes to avoid when working with mole ratios?
Common mistakes include using an unbalanced chemical equation, incorrectly identifying the limiting reactant, neglecting the reaction quotient (Q), and ignoring the effects of temperature and pressure. Always double-check your work and consider all relevant factors for accurate mole ratio calculations.
9.9. How are mole ratios used in titrations?
In titrations, mole ratios are used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The stoichiometry of the reaction between the titrant and the analyte is used to calculate the number of moles of the unknown substance, and thus its concentration.
9.10. Can mole ratios be used to predict the yield of a reaction?
Yes, mole ratios can be used to predict the theoretical yield of a reaction. By identifying the limiting reactant and using the mole ratio between the limiting reactant and the desired product, the maximum amount of product that can be formed can be calculated.
This comprehensive guide enhances your understanding of mole ratios, their applications, and their importance in chemistry. Remember to visit compare.edu.vn for more detailed comparisons and expert insights to help you excel in your chemical studies and applications.
