Comparing Sedgwick-Rafter cell and haemocytometer techniques offers valuable insights for accurate cell counting. This comprehensive comparison, provided by COMPARE.EDU.VN, explores their methodologies, applications, and limitations. By understanding the nuances of each method, researchers and analysts can choose the most appropriate technique for their specific needs, ensuring reliable results in cell density determination. This detailed analysis covers various aspects, including sample preparation, equipment, counting procedures, and data interpretation, empowering informed decisions for precise cell quantification and leveraging alternative cell counting methods.
1. What Are Sedgewick-Rafter Cells and How Are They Used?
Sedgewick-Rafter cells are specialized microscope slides designed for counting microorganisms in liquid samples, but their use has some limits. Their primary application lies in enumerating algae, plankton, and other microorganisms in water samples. The Sedgewick-Rafter cell consists of a rectangular chamber with defined dimensions, typically 50 mm long, 20 mm wide, and 1 mm deep, creating a precise volume of 1 mL. This known volume simplifies cell density calculation.
1.1 Key Features of Sedgewick-Rafter Cells
- Chamber Volume: The 1 mL volume aids in direct cell density determination.
- Grid Pattern: A grid etched onto the chamber facilitates organized counting.
- Applications: Ideal for large microorganisms or low-density samples.
1.2 Procedure for Using Sedgewick-Rafter Cells
- Calibration: Verify the chamber volume using deionized water and weight measurements. Account for variations greater than 5%.
- Sample Preparation: Fix motile cells with Lugol’s solution (acidic for most, basic for coccolithophorids) to prevent movement.
- Chamber Filling: Place the coverslip diagonally and fill the chamber, avoiding bubbles.
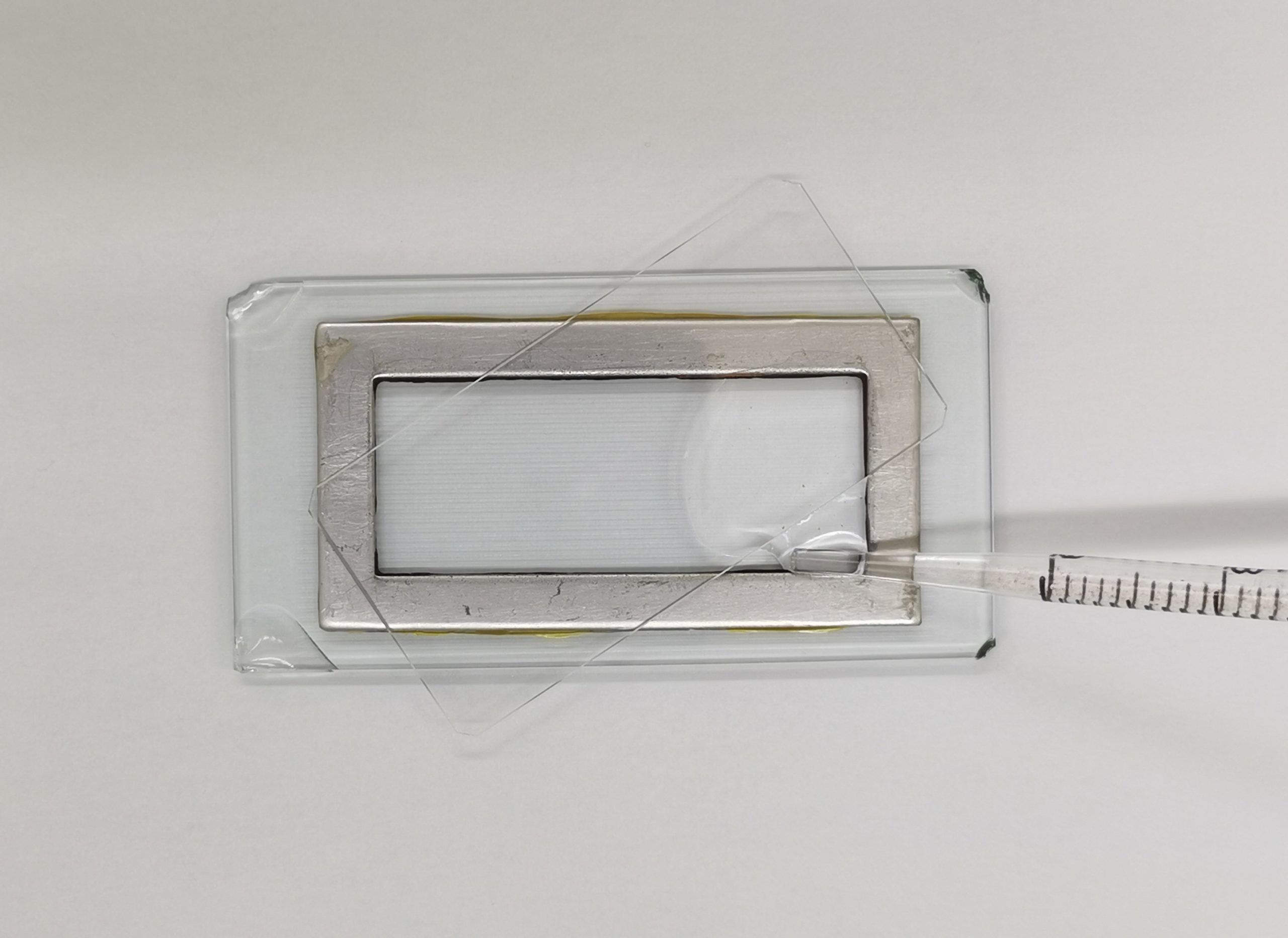 Filling a Sedgwick-Rafter cell with a pipette, showing the diagonal placement of the coverslip to avoid air bubbles
Filling a Sedgwick-Rafter cell with a pipette, showing the diagonal placement of the coverslip to avoid air bubbles
- Settling Time: Allow 15-30 minutes for cells to settle.
- Counting: Count cells in transects, adhering to the convention of including cells touching the top and left-hand side rulings.
- Calculation: Calculate cell concentration (C) using the formula: C = (N x 1000 mm3) / (L x D x W x S).
1.3 Advantages of Sedgewick-Rafter Cells
- Suitable for counting large or colonial organisms.
- Effective for samples with low cell densities (≥ 4 cells / mL).
1.4 Limitations of Sedgewick-Rafter Cells
- Not ideal for small cells or dense cultures without dilution.
- Buoyant cells (e.g., those with gas vacuoles or oil droplets) can be difficult to count.
2. What Are Haemocytometers and How Are They Used?
Haemocytometers are counting chambers used for enumerating cells in liquid samples, offering accuracy for smaller cells. These devices are particularly useful for counting blood cells, but also find application in counting other cell types, including microorganisms. A haemocytometer consists of a thick glass slide with a central counting area etched with a precise grid of known dimensions. This grid allows for accurate cell counting within a defined volume.
2.1 Key Features of Haemocytometers
- Grid Pattern: A precise grid aids in accurate cell counting.
- Chamber Depth: Typically 0.1 mm, providing a defined volume.
- Applications: Best suited for small cells and dense cultures.
2.2 Procedure for Using Haemocytometers
- Preparation: Ensure the haemocytometer and coverslip are clean and dry.
- Assembly: Place the coverslip over the counting area, ensuring proper contact.
- Sample Loading: Introduce the sample into the chamber via capillary action.
- Settling Time: Allow cells to settle for a few minutes.
- Counting: Count cells within specific squares of the grid, adhering to defined counting rules.
- Calculation: Calculate cell density based on the number of cells counted and the volume of the counting area.
2.3 Advantages of Haemocytometers
- Suitable for counting small cells (5-50 µm).
- Ideal for dense cultures where Sedgewick-Rafter cells may be unsuitable.
- Provides accurate cell counts with proper technique.
2.4 Limitations of Haemocytometers
- May not be suitable for large cells or colonial organisms.
- Requires careful technique to avoid errors in counting.
3. Key Differences Between Sedgewick-Rafter Cells and Haemocytometers
Sedgewick-Rafter cells and haemocytometers both facilitate cell counting, but they cater to different sample types and densities. Understanding their differences is crucial for selecting the appropriate method for specific applications.
3.1 Sample Type and Density
- Sedgewick-Rafter Cells: Best for large cells or colonies and low-density samples (≥ 4 cells / mL).
- Haemocytometers: More suitable for small cells (5-50 µm) and dense cultures.
3.2 Chamber Volume and Dimensions
- Sedgewick-Rafter Cells: Larger chamber volume (1 mL) with dimensions of 50 mm x 20 mm x 1 mm.
- Haemocytometers: Smaller chamber depth (0.1 mm) and a grid of defined dimensions.
3.3 Counting Procedure
- Sedgewick-Rafter Cells: Counting is typically done along transects, covering multiple squares.
- Haemocytometers: Counting involves specific squares within the grid, following precise rules.
3.4 Applications
- Sedgewick-Rafter Cells: Commonly used for counting algae and plankton in water samples.
- Haemocytometers: Frequently used for counting blood cells and other small cell types in various applications.
3.5 Practical Considerations
- Sedgewick-Rafter Cells: Variations in chamber volume require calibration.
- Haemocytometers: Proper technique is essential to avoid counting errors.
4. Factors to Consider When Choosing a Method
Choosing between Sedgewick-Rafter cells and haemocytometers depends on several factors related to the sample and the desired accuracy. Consider the following aspects to make an informed decision.
4.1 Cell Size and Density
- Cell Size: For cells larger than 50 µm, Sedgewick-Rafter cells are more suitable due to their larger chamber volume. Haemocytometers are ideal for smaller cells (5-50 µm).
- Cell Density: In low-density samples, Sedgewick-Rafter cells are advantageous. For dense cultures, haemocytometers provide better accuracy.
4.2 Sample Type
- Algal Cultures: Sedgewick-Rafter cells are commonly used for counting algae and plankton in water samples.
- Blood Cells: Haemocytometers are the standard for counting blood cells and other small cell types.
4.3 Motility of Cells
- Motile Cells: Regardless of the counting chamber, motile cells should be fixed with Lugol’s solution to ensure accurate counts.
4.4 Required Accuracy
- High Accuracy: Haemocytometers, when used with proper technique, offer higher accuracy for cell counting.
- General Estimates: Sedgewick-Rafter cells provide reasonable estimates for larger cells or colonies in less dense samples.
4.5 Equipment and Training
- Equipment Availability: Consider the availability of the equipment and the training required for each method.
- Ease of Use: Sedgewick-Rafter cells are generally easier to use for large or colonial organisms, while haemocytometers require more precision.
5. Step-by-Step Guide to Using a Sedgewick-Rafter Cell
To ensure accurate cell counts with a Sedgewick-Rafter cell, follow this step-by-step guide. Proper preparation and technique are crucial for reliable results.
5.1 Calibration of the Chamber
- Measure Dimensions: Verify the chamber dimensions (50 mm x 20 mm x 1 mm).
- Fill with Water: Fill the chamber with deionized water.
- Weight Measurement: Measure the weight of the water-filled chamber.
- Calculate Volume: Calculate the volume based on weight and compare it to the nominal volume of 1 mL.
- Account for Variations: If the variation is greater than 5%, use a calibration factor in subsequent calculations.
5.2 Sample Preparation
- Fixation: For motile cells, add Lugol’s solution (1-2% Lugol’s solution to 1 mL sample).
- Dilution/Concentration: If necessary, dilute dense cultures or concentrate dilute samples by centrifugation or settling.
- Homogenization: Mix the sample gently but thoroughly before sub-sampling.
5.3 Chamber Filling
- Coverslip Placement: Place the coverslip diagonally across the chamber.
- Filling: Use a wide-tipped pipette to carefully fill the chamber from one corner, avoiding bubbles.
- Adjustment: Adjust the coverslip to cover the chamber completely.
5.4 Settling Time
- Allow Settling: Let the cells settle for 15-30 minutes.
- Check Distribution: Examine the chamber to ensure an even spread of settled cells.
5.5 Counting
- Transect Selection: Choose one or two long transects in the chamber.
- Cell Counting: Count cells in each square along the transect.
- Adherence to Rules: Count only cells touching the top and left-hand side rulings of each square.
- Minimum Counts: Count a minimum of 30 cells or until the average and standard deviation are stable.
5.6 Calculation of Cell Concentration
- Use the Formula: C = (N x 1000 mm3) / (L x D x W x S).
- Variables:
- N = number of cells/colonies counted
- L = length of transect strip (mm)
- W = width of transect strip (mm)
- D = chamber depth (mm)
- S = number of transects counted
- Adjustments: Apply any dilution or concentration factors to the final count.
6. Step-by-Step Guide to Using a Haemocytometer
Using a haemocytometer requires precision to achieve accurate cell counts. Follow this detailed guide for best results.
6.1 Preparation of the Haemocytometer
- Cleaning: Ensure the haemocytometer and coverslip are clean and dry.
- Assembly: Place the coverslip over the counting area, ensuring proper contact.
6.2 Sample Loading
- Mixing: Mix the sample gently but thoroughly before loading.
- Loading Technique: Use a pipette to introduce the sample into the chamber via capillary action.
- Avoid Overfilling: Ensure the chamber is filled without overfilling or introducing air bubbles.
6.3 Settling Time
- Allow Settling: Let the cells settle for a few minutes to ensure they are stationary.
6.4 Counting
- Microscope Setup: Place the haemocytometer on the microscope stage and focus on the grid.
- Counting Rules: Count cells within specific squares of the grid, adhering to defined counting rules.
- Edge Cells: Typically, count cells touching the top and left lines of a square and exclude those touching the bottom and right lines.
- Multiple Squares: Count cells in multiple squares to improve accuracy.
6.5 Calculation of Cell Density
- Determine Volume: Calculate the volume of the counting area based on the dimensions of the grid.
- Use the Formula: Calculate cell density based on the number of cells counted and the volume of the counting area.
- Adjustments: Apply any dilution factors to the final count.
7. Common Mistakes and How to Avoid Them
Avoiding common mistakes is essential for accurate cell counting with both Sedgewick-Rafter cells and haemocytometers. Here are some pitfalls and how to prevent them.
7.1 Uneven Cell Distribution
- Mistake: Cells are not evenly distributed in the chamber, leading to inaccurate counts.
- Solution: Ensure thorough mixing of the sample before loading and allow sufficient settling time.
7.2 Air Bubbles
- Mistake: Air bubbles in the chamber interfere with cell counting.
- Solution: Carefully fill the chamber, avoiding the introduction of air bubbles.
7.3 Improper Settling Time
- Mistake: Not allowing enough time for cells to settle, resulting in moving cells and inaccurate counts.
- Solution: Allow adequate settling time (15-30 minutes for Sedgewick-Rafter cells, a few minutes for haemocytometers).
7.4 Inconsistent Counting Rules
- Mistake: Not following consistent counting rules for cells on the edges of squares.
- Solution: Adhere to a strict convention for counting edge cells (e.g., counting only those touching the top and left lines).
7.5 Calibration Errors
- Mistake: Not calibrating the Sedgewick-Rafter cell or using incorrect calibration factors.
- Solution: Calibrate the chamber regularly and use accurate calibration factors in calculations.
7.6 Sample Preparation Errors
- Mistake: Improper fixation or dilution of the sample.
- Solution: Use the correct fixative and dilution methods for the sample type.
8. Advanced Techniques and Considerations
For more complex or specialized applications, consider these advanced techniques and considerations to enhance the accuracy and reliability of cell counting.
8.1 Use of Stains
- Viability Stains: Use viability stains (e.g., trypan blue) to distinguish between live and dead cells.
- Fluorescent Stains: Employ fluorescent stains for specific cell types or structures.
8.2 Automated Cell Counters
- Advantages: Automated cell counters offer faster and more accurate cell counts compared to manual methods.
- Considerations: Ensure the automated counter is appropriate for the cell type and size range.
8.3 Image Analysis
- Technique: Use image analysis software to automate cell counting from microscopic images.
- Benefits: Reduces human error and allows for detailed analysis of cell morphology.
8.4 Quality Control
- Replicates: Perform multiple counts and calculate the average and standard deviation to assess variability.
- Controls: Use control samples with known cell densities to validate the accuracy of the method.
9. Case Studies: Comparing Applications
Examining specific case studies highlights the practical differences between Sedgewick-Rafter cells and haemocytometers, aiding in method selection.
9.1 Algal Bloom Monitoring
- Scenario: Monitoring algal blooms in a lake with large, colonial algae.
- Best Method: Sedgewick-Rafter cells are ideal due to their ability to count large colonies and low-density samples.
9.2 Cell Culture Density Measurement
- Scenario: Measuring the density of a small cell culture in a laboratory setting.
- Best Method: Haemocytometers provide accurate counts for small cells in dense cultures.
9.3 Water Quality Assessment
- Scenario: Assessing the presence of microorganisms in a water sample.
- Best Method: Sedgewick-Rafter cells are suitable for identifying and counting various microorganisms.
9.4 Blood Cell Counting
- Scenario: Counting blood cells in a clinical laboratory.
- Best Method: Haemocytometers are the standard for accurate blood cell counts.
10. Advantages of Using COMPARE.EDU.VN for Method Selection
COMPARE.EDU.VN offers valuable resources for comparing and selecting the best cell counting method for specific applications. Our platform provides detailed comparisons, step-by-step guides, and expert insights to help you make informed decisions.
10.1 Comprehensive Comparisons
- Detailed Analysis: COMPARE.EDU.VN offers comprehensive comparisons of Sedgewick-Rafter cells and haemocytometers, covering all relevant aspects.
- Objective Evaluations: Our evaluations are objective and based on scientific principles and practical considerations.
10.2 Expert Insights
- Expert Opinions: Gain access to expert opinions and recommendations on the best method for specific applications.
- Practical Tips: Benefit from practical tips and advice to improve the accuracy and reliability of cell counting.
10.3 Step-by-Step Guides
- Easy-to-Follow Instructions: Access step-by-step guides for using Sedgewick-Rafter cells and haemocytometers.
- Visual Aids: Benefit from visual aids and diagrams to enhance understanding.
10.4 Time and Resource Savings
- Efficient Selection: Save time and resources by quickly identifying the most appropriate method for your needs.
- Reduced Errors: Minimize errors by following our expert guidance.
11. Frequently Asked Questions (FAQ)
11.1 When should I use a Sedgewick-Rafter cell instead of a haemocytometer?
Use a Sedgewick-Rafter cell for large cells or colonies, or when working with low-density samples, due to its larger chamber volume and suitability for such specimens.
11.2 Can I use Lugol’s solution for all types of cells?
Lugol’s solution is generally safe, but for microalgae with calcium carbonate scales (like coccolithophorids), use a basic solution instead of the acidic form to avoid destroying the organisms.
11.3 How do I calibrate a Sedgewick-Rafter cell?
Calibrate by filling the chamber with deionized water, measuring its weight, and comparing the calculated volume to the nominal volume. Account for variations greater than 5%.
11.4 What is the settling time for cells in a Sedgewick-Rafter cell?
Allow cells to settle for approximately 15-30 minutes to ensure accurate counts.
11.5 How do I avoid air bubbles when filling a counting chamber?
Place the coverslip diagonally and carefully fill the chamber from one corner, adjusting the coverslip to cover the chamber completely while avoiding air bubbles.
11.6 What should I do if the cell density is too high for accurate counting?
Dilute the sample with a known volume of culture media and apply a dilution factor to the final cell count.
11.7 What are the common mistakes to avoid when using a haemocytometer?
Avoid uneven cell distribution, air bubbles, improper settling time, and inconsistent counting rules to ensure accurate counts.
11.8 Can automated cell counters replace manual counting methods?
Automated cell counters offer faster and more accurate counts, but ensure they are appropriate for the cell type and size range you are working with.
11.9 How can I improve the accuracy of cell counts?
Improve accuracy by using stains to differentiate between live and dead cells, performing multiple counts, and using control samples for validation.
11.10 Where can I find reliable information on cell counting methods?
COMPARE.EDU.VN provides comprehensive comparisons, expert insights, and step-by-step guides to help you select the best cell counting method for your specific needs.
12. Conclusion: Making Informed Decisions with COMPARE.EDU.VN
Choosing the right cell counting method is essential for accurate and reliable results. COMPARE.EDU.VN provides the resources and expertise needed to make informed decisions, whether you are working with Sedgewick-Rafter cells or haemocytometers. By understanding the key differences, advantages, and limitations of each method, you can optimize your cell counting procedures and achieve the best possible outcomes.
Are Sedgewick And Haemocytometer Methods Comparable? While both serve the purpose of cell counting, their comparability depends on the specific application and sample characteristics. Sedgewick-Rafter cells are well-suited for large cells in low-density samples, whereas haemocytometers excel with small cells in dense cultures. By considering factors such as cell size, density, and motility, researchers can determine which method aligns best with their objectives. Ultimately, the choice between Sedgewick-Rafter cells and haemocytometers hinges on achieving accurate and reliable cell counts tailored to the specific research question.
Ready to make a smart choice? Visit COMPARE.EDU.VN today to explore detailed comparisons, expert insights, and step-by-step guides that will help you select the best cell counting method for your specific needs. Don’t leave your results to chance—trust COMPARE.EDU.VN to provide the information you need to succeed. Contact us at: Address: 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090. Website: compare.edu.vn.