Are Carboxylic Acids Compared To Esters distinct chemical entities? Yes, carboxylic acids and esters, while both featuring a carbonyl group, differ significantly in structure, properties, and applications. COMPARE.EDU.VN provides a detailed comparison to understand these crucial differences, enabling informed decisions in various scientific and industrial contexts. Dive into an exploration of their reactivity, synthesis, and natural occurrence, unlocking a deeper understanding of organic chemistry principles and unveiling new insights.
1. What Are Carboxylic Acids?
Carboxylic acids are organic compounds characterized by the presence of a carboxyl group (-COOH) attached to an alkyl or aryl group. This functional group consists of a carbonyl group (C=O) and a hydroxyl group (-OH). Carboxylic acids are widely found in nature and are essential in various biological and industrial processes.
1.1. Structure and Nomenclature of Carboxylic Acids
The general formula of a carboxylic acid is R-COOH, where R represents the alkyl or aryl group. The carboxyl group is always located at the end of the carbon chain.
The nomenclature of carboxylic acids follows specific IUPAC rules:
- Identify the longest continuous carbon chain containing the carboxyl group.
- Replace the “-e” ending of the corresponding alkane with “-oic acid”.
- Number the carbon chain starting with the carboxyl carbon as carbon number 1.
- Name and number any substituents attached to the carbon chain.
For example, ethanoic acid (CH3COOH) is commonly known as acetic acid, and methanoic acid (HCOOH) is known as formic acid.
1.2. Properties of Carboxylic Acids
Carboxylic acids exhibit distinct physical and chemical properties due to the presence of the carboxyl group.
- Physical Properties:
- Polarity: Carboxylic acids are polar molecules due to the electronegativity difference between oxygen and carbon and hydrogen atoms.
- Hydrogen Bonding: They can form hydrogen bonds with each other and with other polar molecules, leading to relatively high boiling points compared to hydrocarbons of similar molecular weight.
- Solubility: Lower molecular weight carboxylic acids are soluble in water due to their ability to form hydrogen bonds with water molecules. Solubility decreases as the carbon chain length increases due to the increasing hydrophobic character.
- Chemical Properties:
- Acidity: Carboxylic acids are weak acids, meaning they only partially dissociate in water to release hydrogen ions (H+). The acidity is attributed to the carboxyl group, where the hydrogen atom of the hydroxyl group can be donated.
- Reactivity: They undergo various chemical reactions, including:
- Neutralization: React with bases to form salts and water.
- Esterification: React with alcohols to form esters.
- Amide Formation: React with amines to form amides.
- Reduction: Can be reduced to alcohols or aldehydes.
1.3. Natural Occurrence and Uses of Carboxylic Acids
Carboxylic acids are abundant in nature. For example:
- Formic acid is found in ant stings.
- Acetic acid is present in vinegar.
- Citric acid is found in citrus fruits.
- Fatty acids such as palmitic acid and stearic acid are components of fats and oils.
Industrially, carboxylic acids are used in:
- Production of polymers: Acetic acid in the production of polyvinyl acetate (PVA).
- Food preservatives: Benzoic acid and sorbic acid.
- Pharmaceuticals: Salicylic acid in aspirin.
2. What Are Esters?
Esters are organic compounds formed by the reaction of a carboxylic acid with an alcohol, with the elimination of water. They are characterized by the presence of an ester group (-COOR’) in their structure, where R and R’ are alkyl or aryl groups. Esters are widely known for their pleasant odors and flavors and are used in perfumes, flavorings, and solvents.
2.1. Structure and Nomenclature of Esters
The general formula of an ester is R-COOR’, where R is the alkyl or aryl group from the carboxylic acid and R’ is the alkyl or aryl group from the alcohol.
The nomenclature of esters involves two parts:
-
Name the alkyl group (R’) derived from the alcohol.
-
Name the acyl group (RCOO-) derived from the carboxylic acid by replacing “-ic acid” with “-ate”.
For example, ethyl acetate (CH3COOCH2CH3) is formed from acetic acid and ethanol, and methyl benzoate (C6H5COOCH3) is formed from benzoic acid and methanol.
2.2. Properties of Esters
Esters exhibit unique physical and chemical properties that distinguish them from carboxylic acids and alcohols.
- Physical Properties:
- Volatility: Esters generally have lower boiling points compared to carboxylic acids and alcohols of similar molecular weight due to the absence of hydrogen bonding.
- Odor: Many esters have pleasant, fruity odors, making them useful in perfumes and flavorings.
- Solubility: Lower molecular weight esters are slightly soluble in water, but solubility decreases with increasing carbon chain length.
- Chemical Properties:
- Hydrolysis: Esters undergo hydrolysis, reacting with water to form a carboxylic acid and an alcohol. This reaction can be acid-catalyzed or base-catalyzed (saponification).
- Transesterification: Esters react with alcohols to exchange the alkoxy group, forming a new ester and a new alcohol.
- Reduction: Esters can be reduced to alcohols using reducing agents such as lithium aluminum hydride (LiAlH4).
2.3. Natural Occurrence and Uses of Esters
Esters are prevalent in nature and have numerous applications in various industries.
- Natural Occurrence:
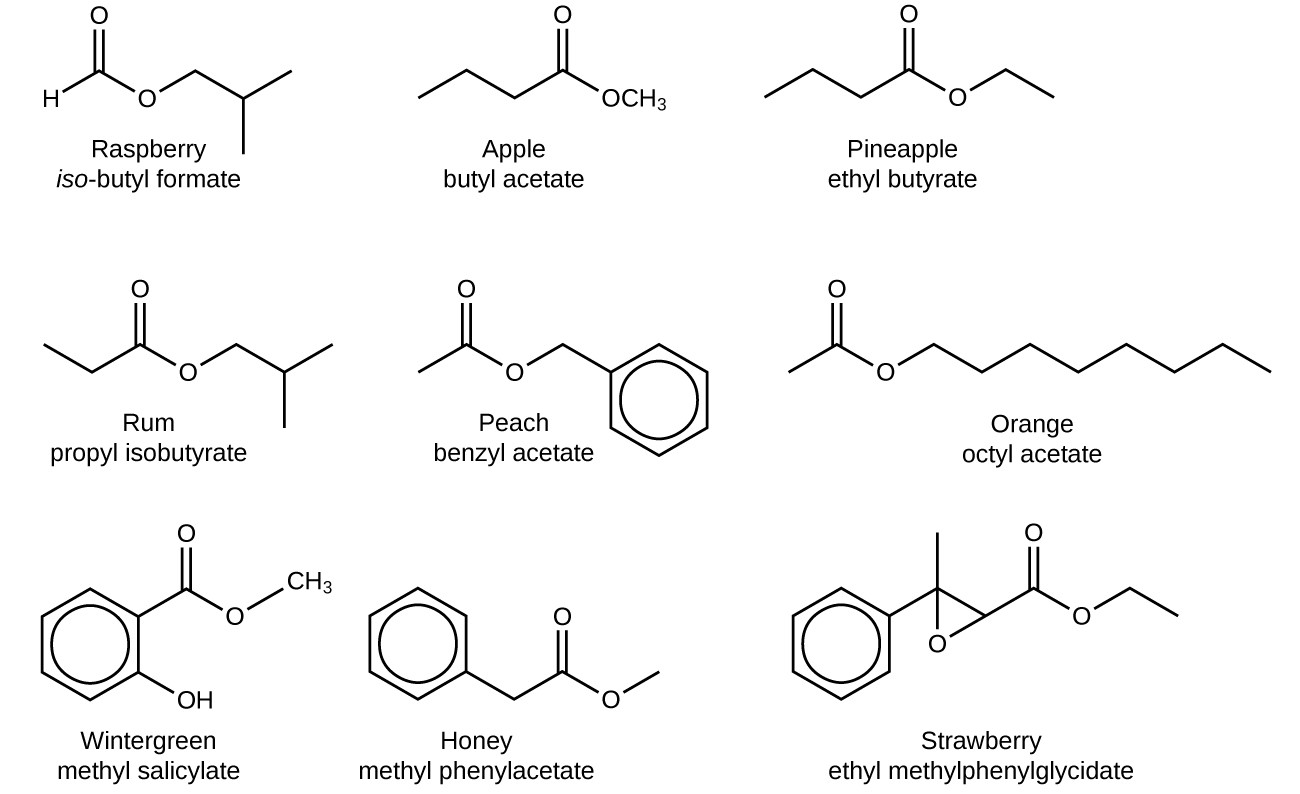
- Fruits: Many fruits owe their characteristic odors and flavors to esters, such as ethyl butanoate in pineapple and benzyl acetate in jasmine.
- Fats and Oils: Triglycerides, which are esters of glycerol and fatty acids, are major components of fats and oils.
- Industrial Uses:
- Solvents: Ethyl acetate and butyl acetate are common solvents in paints, coatings, and adhesives.
- Perfumes and Flavorings: Various esters are used to impart specific scents and flavors in perfumes, cosmetics, and food products.
- Pharmaceuticals: Some esters are used as prodrugs, which are converted into active drugs in the body through hydrolysis.
3. Key Differences: Are Carboxylic Acids Compared to Esters?
While both carboxylic acids and esters contain a carbonyl group, their structures and properties differ significantly. A direct comparison highlights these differences:
3.1. Structural Differences
| Feature | Carboxylic Acids (R-COOH) | Esters (R-COOR’) |
|---|---|---|
| Functional Group | Carboxyl (-COOH) | Ester (-COOR’) |
| Composition | Carbonyl + Hydroxyl | Acyl + Alkoxy |
| Bonding | C=O, C-OH | C=O, C-O-C |
| Hydrogen | Directly bonded to Oxygen | Absent from Oxygen |

Carboxylic acids have a hydroxyl group directly attached to the carbonyl carbon, whereas esters have an alkoxy group (OR’) in place of the hydroxyl group.
3.2. Property Differences
| Property | Carboxylic Acids | Esters |
|---|---|---|
| Acidity | Weakly acidic | Neutral |
| Boiling Point | Higher | Lower |
| Hydrogen Bonding | Stronger | Weaker |
| Odor | Pungent/Irritating | Pleasant/Fruity |
| Reactivity | More reactive | Less reactive |
3.3. Reactivity Comparison
Carboxylic acids and esters undergo different types of reactions due to their structural differences.
- Acidity: Carboxylic acids exhibit acidic behavior due to the presence of the hydroxyl group, while esters are neutral.
- Hydrolysis: Esters undergo hydrolysis to form carboxylic acids and alcohols, whereas carboxylic acids do not undergo hydrolysis.
- Esterification: Carboxylic acids react with alcohols to form esters, whereas esters can undergo transesterification to form different esters.
4. Synthesis Methods: Carboxylic Acids and Esters
The synthesis of carboxylic acids and esters involves distinct chemical pathways, each leveraging specific reactants and conditions to achieve the desired product. Understanding these methods provides insight into their chemical behavior and industrial production.
4.1. Synthesis of Carboxylic Acids
Carboxylic acids can be synthesized through various methods, including:
-
Oxidation of Primary Alcohols and Aldehydes: Primary alcohols and aldehydes can be oxidized to carboxylic acids using oxidizing agents such as potassium permanganate (KMnO4) or chromic acid (H2CrO4).
R-CH2OH -> R-CHO -> R-COOH -
Hydrolysis of Nitriles: Nitriles (R-CN) can be hydrolyzed under acidic or basic conditions to form carboxylic acids.
R-CN + 2H2O -> R-COOH + NH3 -
Grignard Reagents: Carboxylation of Grignard reagents (R-MgX) with carbon dioxide (CO2) followed by acidification yields carboxylic acids.
R-MgX + CO2 -> R-COOMgX -> R-COOH
4.2. Synthesis of Esters
Esters are typically synthesized through the esterification of carboxylic acids with alcohols, a process that can be acid-catalyzed.
-
Fischer Esterification: Carboxylic acids react with alcohols in the presence of an acid catalyst (e.g., sulfuric acid) to form esters and water.
R-COOH + R'OH -> R-COOR' + H2O -
Acylation of Alcohols: Alcohols react with acyl chlorides or acid anhydrides to form esters.
R-COCl + R'OH -> R-COOR' + HCl(RCO)2O + R'OH -> R-COOR' + RCOOH -
Transesterification: Esters react with alcohols to exchange the alkoxy group, forming a new ester and a new alcohol.
R-COOR' + R"OH -> R-COOR" + R'OH
5. Applications Across Industries
Carboxylic acids and esters find extensive applications across various industries, each capitalizing on their unique properties and functionalities.
5.1. Carboxylic Acids in Industrial Applications
Carboxylic acids are integral to numerous industrial processes and products:
- Polymer Production: Acetic acid is a key component in the production of polyvinyl acetate (PVA), used in adhesives, coatings, and textiles. Acrylic acid is used in the production of acrylic polymers, which are used in paints, plastics, and superabsorbent polymers.
- Food Preservation: Benzoic acid and sorbic acid are widely used as food preservatives due to their antimicrobial properties, preventing the growth of bacteria, yeast, and molds in food products.
- Pharmaceuticals: Salicylic acid is a precursor in the synthesis of aspirin (acetylsalicylic acid), a common analgesic and anti-inflammatory drug. Various other carboxylic acid derivatives are used in drug synthesis.
- Cleaning Agents: Citric acid and acetic acid are used in cleaning agents and descalers due to their ability to dissolve mineral deposits and remove stains.
- Textile Industry: Acetic acid is used in textile dyeing and finishing processes to adjust pH levels and improve dye uptake.
- Rubber Production: Stearic acid is used as a lubricant and processing aid in the production of rubber products, improving the texture and flexibility of the rubber.
5.2. Esters in Industrial Applications
Esters are valued for their versatility and are employed in a wide array of applications:
- Solvents: Ethyl acetate and butyl acetate are common solvents in paints, coatings, and adhesives due to their ability to dissolve a variety of organic compounds. They are also used in the production of lacquers and varnishes.
- Perfumes and Flavorings: Esters contribute characteristic scents and flavors to perfumes, cosmetics, and food products. For example, ethyl butanoate is used in pineapple flavoring, and benzyl acetate is used in jasmine perfumes.
- Plasticizers: Phthalate esters are used as plasticizers in the production of polyvinyl chloride (PVC), increasing its flexibility and durability. These esters are added to PVC to make it more pliable for use in products such as vinyl flooring, cables, and synthetic leather.
- Pharmaceuticals: Esters are used as prodrugs, which are converted into active drugs in the body through hydrolysis. This approach can improve drug bioavailability, stability, and taste.
- Biodiesel Production: Transesterification of vegetable oils with methanol or ethanol produces biodiesel, an alternative fuel source. This process converts triglycerides into methyl or ethyl esters of fatty acids.
- Textile Industry: Esters are used as softening agents and lubricants in the textile industry, improving the texture and feel of fabrics.
- Cosmetics: Various esters are used in cosmetic formulations as emollients, fragrances, and solvents. They contribute to the texture, scent, and application properties of cosmetic products.
6. Comparative Analysis in Detail
A detailed comparative analysis provides a comprehensive understanding of the differences and similarities between carboxylic acids and esters.
6.1. Structure and Bonding
- Carboxylic Acids: The carboxyl group (-COOH) consists of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom. The carbon atom is sp2 hybridized, resulting in a trigonal planar geometry around the carbonyl carbon. The hydroxyl group is capable of forming hydrogen bonds, which significantly influences the physical and chemical properties of carboxylic acids.
- Esters: The ester group (-COOR’) is formed by replacing the hydroxyl hydrogen of a carboxylic acid with an alkyl or aryl group. The carbonyl carbon is also sp2 hybridized, with a trigonal planar geometry. Esters lack the direct hydrogen bonding capability of carboxylic acids, resulting in lower boiling points and different solubility characteristics.
6.2. Physical Properties
| Property | Carboxylic Acids | Esters |
|---|---|---|
| Boiling Point | Higher boiling points due to strong hydrogen bonding between molecules. | Lower boiling points compared to carboxylic acids due to weaker intermolecular forces (dipole-dipole). |
| Solubility | Lower molecular weight carboxylic acids are soluble in water due to hydrogen bonding. | Lower molecular weight esters are slightly soluble in water, but solubility decreases with chain length. |
| Odor | Often have pungent, irritating odors (e.g., acetic acid). | Many have pleasant, fruity odors (e.g., ethyl acetate, butyl butanoate). |
| Volatility | Less volatile compared to esters due to stronger intermolecular forces. | More volatile compared to carboxylic acids due to weaker intermolecular forces. |
6.3. Chemical Reactivity
| Reaction | Carboxylic Acids | Esters |
|---|---|---|
| Acidity | Weak acids; donate a proton (H+) from the hydroxyl group. | Neutral; do not exhibit acidic or basic properties. |
| Esterification | React with alcohols to form esters (Fischer esterification). | Undergo transesterification, reacting with alcohols to form different esters. |
| Hydrolysis | Relatively resistant to hydrolysis under neutral conditions. | Undergo hydrolysis (acid- or base-catalyzed) to form carboxylic acids and alcohols. |
| Reduction | Can be reduced to primary alcohols using strong reducing agents like lithium aluminum hydride (LiAlH4). | Can be reduced to alcohols using strong reducing agents (LiAlH4) or undergo reduction to aldehydes under specific conditions. |
| Saponification | React with bases to form salts (carboxylic acid salts). | Undergo saponification (base-catalyzed hydrolysis) to form carboxylic acid salts and alcohols. |
| Amide Formation | React with amines to form amides. | Less reactive towards amines compared to carboxylic acids but can form amides under specific conditions (e.g., with ammonia or primary amines). |
6.4. Synthesis Routes
| Synthesis Method | Carboxylic Acids | Esters |
|---|---|---|
| Oxidation | Oxidation of primary alcohols or aldehydes using oxidizing agents like KMnO4 or CrO3. | Not typically synthesized directly by oxidation (usually synthesized from carboxylic acids). |
| Hydrolysis | Hydrolysis of nitriles (R-CN) under acidic or basic conditions. | Formed through the hydrolysis of other esters or under specific reaction conditions. |
| Grignard Reaction | Reaction of Grignard reagents (R-MgX) with carbon dioxide (CO2) followed by acidification. | Not directly synthesized via Grignard reactions. |
| Esterification | Not formed directly; precursors to esters. | Fischer esterification: Reaction of carboxylic acids with alcohols in the presence of an acid catalyst. |
| Acylation | Not formed directly. | Acylation of alcohols with acyl chlorides or acid anhydrides. |
| Transesterification | Not formed directly. | Transesterification: Reaction of an ester with an alcohol to exchange alkoxy groups. |
7. Environmental and Safety Considerations
When working with carboxylic acids and esters, it is crucial to consider their environmental impact and ensure safe handling.
7.1. Environmental Impact
- Carboxylic Acids: Some carboxylic acids, such as acetic acid and formic acid, can contribute to environmental pollution if released in large quantities. Acetic acid, a component of vinegar, can lower the pH of water bodies, affecting aquatic life. Formic acid, used in various industrial processes, can also be harmful if not properly managed. Proper waste management and treatment are essential to minimize their environmental impact.
- Esters: Certain esters, particularly phthalates, have raised environmental concerns due to their widespread use as plasticizers. Phthalates can leach from plastic products and contaminate water and soil, posing risks to human health and wildlife. Biodegradable esters, such as those derived from renewable resources, are gaining attention as more environmentally friendly alternatives.
7.2. Safety Precautions
- Carboxylic Acids:
- Corrosivity: Concentrated carboxylic acids, such as acetic acid and formic acid, are corrosive and can cause skin and eye irritation or burns. Appropriate personal protective equipment (PPE), including gloves, safety goggles, and lab coats, should be worn when handling these substances.
- Flammability: Some carboxylic acids, such as acetic acid, are flammable and should be kept away from open flames and sources of ignition.
- Inhalation Hazards: Inhalation of carboxylic acid vapors can cause respiratory irritation. Use in well-ventilated areas or with respiratory protection.
- Esters:
- Flammability: Many esters are flammable and can form explosive mixtures with air. Store in tightly closed containers away from heat, sparks, and open flames.
- Irritation: Some esters can cause skin and eye irritation. Wear appropriate PPE, such as gloves and safety goggles, when handling these substances.
- Vapor Hazards: Inhalation of ester vapors can cause dizziness, drowsiness, and respiratory irritation. Use in well-ventilated areas or with respiratory protection.
7.3. Handling and Storage
- Carboxylic Acids:
- Store in tightly closed containers in a cool, dry, and well-ventilated area.
- Keep away from incompatible materials, such as strong bases and oxidizing agents.
- Use proper labeling to identify the contents and hazards.
- Esters:
- Store in tightly closed containers in a cool, dry, and well-ventilated area.
- Keep away from heat, sparks, and open flames.
- Avoid contact with strong acids, bases, and oxidizing agents.
- Use proper grounding and bonding techniques to prevent static electricity buildup when transferring flammable esters.
8. Recent Advances and Research
Recent advancements in the chemistry of carboxylic acids and esters are expanding their applications and improving their sustainability.
8.1. Sustainable Synthesis Methods
- Biocatalysis: Enzymes are increasingly used as biocatalysts in the synthesis of carboxylic acids and esters, offering environmentally friendly alternatives to traditional chemical methods. Enzymes can catalyze reactions under mild conditions, reducing the use of hazardous chemicals and energy consumption.
- Renewable Feedstocks: Research focuses on using renewable feedstocks, such as biomass and waste materials, to produce carboxylic acids and esters. This approach reduces reliance on fossil fuels and promotes a circular economy.
8.2. Advanced Materials
- Biodegradable Polymers: Carboxylic acid and ester-based polymers are being developed as biodegradable alternatives to conventional plastics. These polymers can degrade naturally in the environment, reducing plastic waste and pollution.
- Smart Materials: Carboxylic acid and ester derivatives are used in the development of smart materials with stimuli-responsive properties. These materials can change their properties in response to external stimuli, such as pH, temperature, or light, enabling applications in drug delivery, sensors, and actuators.
8.3. Pharmaceutical Applications
- Prodrug Design: Esters are widely used in prodrug design to improve the bioavailability, stability, and targeting of drugs. Ester prodrugs can be designed to release the active drug molecule under specific conditions, such as enzymatic hydrolysis in the target tissue.
- Drug Delivery Systems: Carboxylic acid and ester-based nanoparticles and microparticles are being developed as drug delivery systems. These systems can encapsulate drugs and release them in a controlled manner, improving therapeutic efficacy and reducing side effects.
9. Practical Examples and Case Studies
Examining practical examples and case studies illustrates the real-world applications and significance of carboxylic acids and esters.
9.1. Case Study: Production of Biodiesel
Biodiesel is a renewable fuel produced through the transesterification of vegetable oils, animal fats, or recycled grease with methanol or ethanol. This process converts triglycerides (esters of glycerol and fatty acids) into methyl or ethyl esters of fatty acids, known as biodiesel.
-
Process:
- Feedstock Preparation: Vegetable oils or animal fats are pretreated to remove impurities and water.
- Transesterification: The oil is mixed with an alcohol (methanol or ethanol) and a catalyst (sodium hydroxide or potassium hydroxide). The catalyst facilitates the reaction between the triglycerides and alcohol, breaking the ester bonds and forming fatty acid methyl or ethyl esters.
- Separation: The reaction mixture is allowed to settle, separating the biodiesel from glycerol and excess alcohol.
- Purification: The biodiesel is washed to remove residual catalyst, alcohol, and glycerol, followed by drying to remove any remaining water.
-
Benefits:
- Renewable Resource: Biodiesel is derived from renewable resources, reducing reliance on fossil fuels.
- Reduced Emissions: Biodiesel combustion produces lower emissions of particulate matter, carbon monoxide, and hydrocarbons compared to petroleum diesel.
- Biodegradability: Biodiesel is biodegradable and less toxic than petroleum diesel, reducing the environmental impact of spills.
9.2. Case Study: Aspirin Synthesis
Aspirin (acetylsalicylic acid) is a widely used analgesic, antipyretic, and anti-inflammatory drug synthesized by the esterification of salicylic acid with acetic anhydride.
-
Process:
- Reaction: Salicylic acid reacts with acetic anhydride in the presence of an acid catalyst (sulfuric acid or phosphoric acid).
- Esterification: The hydroxyl group of salicylic acid reacts with acetic anhydride, forming acetylsalicylic acid (aspirin) and acetic acid.
- Crystallization: Water is added to the reaction mixture to precipitate aspirin as crystals.
- Purification: The aspirin crystals are filtered, washed, and dried to obtain pure acetylsalicylic acid.
-
Significance:
- Pain Relief: Aspirin is effective in relieving mild to moderate pain, such as headaches, muscle aches, and arthritis.
- Anti-inflammatory: Aspirin reduces inflammation by inhibiting the production of prostaglandins, inflammatory mediators.
- Cardiovascular Protection: Low-dose aspirin is used to prevent blood clot formation, reducing the risk of heart attacks and strokes in high-risk individuals.
10. FAQ Section
| Question | Answer |
|---|---|
| What is the main difference between carboxylic acids and esters? | Carboxylic acids contain a carboxyl group (-COOH), while esters contain an ester group (-COOR’). The carboxyl group includes a hydroxyl group, which contributes to acidity and hydrogen bonding properties not found in esters. |
| Are carboxylic acids stronger acids than esters? | Carboxylic acids are weak acids, while esters are neutral compounds and do not exhibit acidic properties. |
| How are esters synthesized from carboxylic acids? | Esters are commonly synthesized from carboxylic acids through Fischer esterification, which involves reacting a carboxylic acid with an alcohol in the presence of an acid catalyst. |
| What makes esters have pleasant odors? | The pleasant odors of esters are due to their volatility and molecular structure, which allows them to easily evaporate and interact with olfactory receptors in the nose. |
| Can esters be converted back into carboxylic acids? | Yes, esters can be converted back into carboxylic acids through hydrolysis, which can be acid-catalyzed or base-catalyzed (saponification). |
| Why do carboxylic acids have higher boiling points? | Carboxylic acids have higher boiling points than esters due to the strong hydrogen bonding between their molecules, which requires more energy to overcome. |
| What are some common uses of carboxylic acids? | Carboxylic acids are used in the production of polymers, food preservatives, pharmaceuticals, and cleaning agents. |
| What are some common uses of esters? | Esters are used as solvents, perfumes, flavorings, plasticizers, and in the production of biodiesel and pharmaceuticals. |
| Are esters more reactive than carboxylic acids? | No, carboxylic acids are generally more reactive than esters due to the presence of the hydroxyl group, which makes them more susceptible to various chemical reactions. |
| How do the properties of esters affect their applications? | The properties of esters, such as their volatility, pleasant odors, and solvent capabilities, make them suitable for a wide range of applications in the flavor, fragrance, and solvent industries. |
Choosing between different chemical compounds can be a complex task. At COMPARE.EDU.VN, we understand the need for clear, objective comparisons to help you make informed decisions. Whether you’re a student, a professional, or simply curious, our comprehensive guides provide the insights you need.
Ready to explore more comparisons and unlock a world of knowledge? Visit COMPARE.EDU.VN today and discover the power of informed decision-making. Our team of experts is dedicated to providing you with the most accurate and up-to-date information. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or via WhatsApp at +1 (626) 555-9090. Start comparing now at compare.edu.vn!