Introduction
The animal kingdom, both past and present, showcases incredible diversity. Among extinct creatures, Batrachotomus kupferzellensis stands out as a unique example without a perfect modern counterpart. This long-limbed archosaur, belonging to the crocodile lineage (Pseudosuchia clade) from the Middle Triassic period in Germany, possessed a large head and substantial tail reminiscent of contemporary crocodiles. However, its more upright limb posture bore similarities to modern cursorial mammals. This raises a fundamental question: was Batrachotomus locomotion more akin to mammals, modern crocodiles, or something entirely distinct? Understanding how extinct archosaurs moved, especially when compared to their living relatives like crocodiles, provides crucial insights into the evolution of terrestrial locomotion.
Bonaparte (1984) suggested that the erect limbs, along with an elongated pubis and ischium, in “rauisuchids” (a group encompassing Batrachotomus) were adaptations for parasagittal locomotion. This adaptation may have been crucial for their survival during the Middle-Late Triassic faunal turnover. Parrish (1986) further hypothesized that the upright limbs of “rauisuchians” and other archosaurs enhanced their agility on land, drawing parallels to mammals. While acknowledging the similarities in hindlimb plantigrady and crurotarsal ankle structure between these archosaurs and modern crocodiles, Parrish pointed out a key difference. The ankle reorganization in archosaurs led to symmetrical plantarflexor action, resulting in simple ankle plantarflexion, unlike the lateral rotation combined with plantarflexion observed in crocodiles and lizards. Nesbitt et al. (2013) interpreted plantigrady and the extended calcaneal tuber as adaptations for generating high power rather than achieving high speed. Despite the consensus that Batrachotomus employed parasagittal and erect locomotion as a quadruped (Bishop et al., 2020), the specific types of gaits it might have utilized remain unexplored.
Studies on cursorial, quadrupedal mammals have revealed that gait selection is often driven by work-based optimization within simplified parasagittal models. Trajectory optimization, in particular, offers a method for numerically optimizing continuous motion over time. Xi et al. (2016) successfully used trajectory optimization to replicate the four-beat walk and trot commonly observed in mammals, using a planar model. The optimization of galloping was later achieved by incorporating a compliant torso into the model (Yesilevskiy et al., 2018). By minimizing mechanical work in parasagittal models, Usherwood (2020) and Polet (2021b) demonstrated that the body’s pitch moment of inertia (normalized as the Murphy number) is a significant factor in determining whether mammals trot or not.
Remarkably, precise anatomical details are often not essential for biomechanical models to produce solutions comparable to real animals. For instance, a model featuring a rigid body element, massless prismatic legs, and no elastic components accurately simulated walking and trotting in dogs. This model successfully matched gait transition speed, duty factor changes with speed, ground reaction force profiles, and limb phase with reasonable accuracy by minimizing a cost function that combined limb work with a penalty for rapid force changes (Polet and Bertram, 2019). Such simplified models can be particularly valuable in paleontological research, where soft-tissue details like muscle geometry, fiber length, and tendon length are typically not preserved. Determining the gaits of fossil organisms from trackways is often challenging, although some approaches have shown promise (see Nyakatura et al., 2019; Vincelette, 2021).
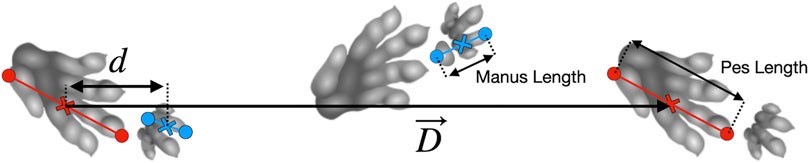
Fossil trackways from the Triassic period, classified under the ichnotaxa Isochirotherium and Brachychirotherium, are potentially attributable to Batrachotomus or a closely related species (Petti et al., 2009; Diedrich, 2015; Apesteguía et al., 2021; Klein and Lucas, 2021). These trackways provide valuable information on the approximate size of the trackmaker and gait parameters, including track phase (defined as the ipsilateral pes to manus displacement divided by stride length, Figure 1). However, the exact gait employed by these ancient trackmakers remains unclear.
FIGURE 1. Schematic of a Brachychirotherium trackway, illustrating parameter definitions. Footprint measurements extend from the caudal-most point to the tip of digit III, or digit II in Brachychirotherium manus. The caudal side of digit V was used when available (e.g., the second manus in the diagram). The manus–pes distance is calculated between midpoints along the stride vector, D→; the track phase is then d/D. Trackway adapted from Wikimedia Commons (2010), used under a CC BY-SA 3.0 license.
A biomechanical model can offer quantitative predictions of the track phase based on stride length and trackmaker size. If the model accurately predicts the track phase, it not only supports the use of a specific gait but also provides insights into other aspects of the trackmaker’s locomotion that are not directly preserved in fossil trackways. This approach allows for a direct comparison of archosaur locomotion to that of modern crocodiles and other quadrupeds.
Methods
Planar Model and Optimization Scheme
The parasagittal, planar model used in this study is based on the work of Polet and Bertram (2019) and Polet (2021b). It features a rigid trunk and massless legs that exert force along an axis connecting the footfall location to the acetabulum or glenoid over time (Figure 2). Feet are modeled as simple points, actuator force is instantly reflected in ground reaction force, and sliding friction is assumed to be infinite. The center of mass (COM) is situated along the glenoacetabular axis, and the model is provided with the pitch moment of inertia (MOI), stride length (D), and average horizontal speed.
FIGURE 2. Simplified quadrupedal model used for simulations, superimposed on the skeletal model used for length measurements. White circles indicate the radius of gyration from the center of mass (x). Only two of the four legs (red and blue lines) are depicted.
The optimization process minimizes an objective function J, which combines axial limb work with a regularization term that penalizes rapid changes in force, as described by the equation:
J=∑i∫0TFiL˙+cFi2˙dt,(1)
Here, Fi represents the axial force of the ith limb, L is the axial limb length, c is the force-rate penalty parameter, and 0 ≤t ≤T is the time duration of a stride. The force-rate penalty ensures continuity in force and velocity, preventing abrupt changes like collisions. However, solutions approaching impulsive forces (quasi-collisions with sharp force peaks) can emerge when force-rate penalties are low.
In the optimization scheme, c is normalized as c′=cMgLBT, where LB is the glenoacetabular distance, M is the body mass, and g is the acceleration due to gravity (Polet and Bertram, 2019). Dimensionless variables are denoted with a prime symbol throughout this paper. Values of c′ reported here are for UH′=0.4 (UH′=U/gH, where U is average horizontal speed, g is acceleration due to gravity, and H is hip height) and were scaled appropriately according to T (to maintain the same c).
Force rates serve as controls in the model, while forces, velocities, and positions are states. A leg-impulse state is incorporated to prevent simultaneous cranial and caudal contact of a single leg. Footfall positions are parameters and decision variables for optimization. Additional relaxation parameters and slack variables are included as controls to regularize complementarity conditions (see Polet and Bertram, 2019 for further details).
Given the symmetry of the gaits of interest, simulations are conducted over a half cycle (time from 0 to T/2). Initial and final COM horizontal positions are set to 0 and D/2, respectively. A periodicity constraint is enforced, ensuring that body kinematics are identical at t=0 and T/2, and left-side forces at t=0 equal right-side forces at t=T/2. The vertical positions of hips and shoulders are constrained to be above ground, and torso orientation (relative to horizontal) is limited to [−π/2,π/2]. Bounds on other parameters, states, and controls are set sufficiently wide to be effectively unconstrained. This allows the optimizer to select any symmetrical contact sequence and duty factor for a given speed and stride length.
Optimal gaits are identified using trajectory optimization with direct collocation, employing GPOPS-II (v. 2.3) (Patterson and Rao, 2014) and SNOPT (v. 7.5) (Gill et al., 2005; Gill et al., 2015) in MATLAB 2020b. For each parameter combination, the best local optimum is selected from a minimum of 50 random initial guesses. Custom software for generating symmetrical solutions is publicly available on GitHub (Polet, 2021a).
In the baseline unconstrained scenario, different force-rate penalties were compared, allowing the optimizer to choose the track phase. In constrained cases, the optimizer was forced to use track phases matching fossil trackway trends. Sensitivity analysis was also performed by increasing forelimb length while keeping the track phase unconstrained. Due to the planar nature of the model, diagonal and lateral sequence gaits are equivalent. The ipsilateral limb phase (ϕL, time from hindlimb contact to ipsilateral forelimb contact divided by stride period) from dog model optimization results was modulated to the range 0<ϕL<0.6, while for the Batrachotomus model, it was modulated to 0.4 <ϕL<1.
Morphology: Domestic Dogs and Batrachotomus
Model track phase predictions were validated against gait and footfall data from Belgian Malinois dogs (Maes et al., 2008), using body proportions from Polet and Bertram (2019) and MOI from Polet (2021b). Dimensions and inertial properties of Batrachotomus were derived from a volumetric model (Bishop et al., 2020), which was based on the skeletal reconstruction at the Staatliches Museum für Naturkunde Stuttgart. Although manus and pes material for this species is fragmented, the autopodia were partially reconstructed by museum staff based on closely related species. We assumed this reconstructed autopodial morphology is sufficiently accurate for our purposes. Gower and Schoch (2009) noted that specimen SMNS 90018 preserves fragmentary metacarpals and phalanges of the right manus, and “four metatarsals, several phalanges, and a few distally incomplete unguals,” with only the fifth metatarsal’s identity being certain. However, the preserved autopodial morphology aligns with: (1) the mounted museum display specimen’s morphology, featuring a relatively small manus compared to the pes; and (2) the general autopodial morphology of other “rauisuchian”-grade Pseudosuchia, also with smaller manus relative to pes. The model was posed in an approximate standing posture to determine leg lengths and pitch MOI about the COM (Figure 2; Table 1). The ratio of hip-to-COM distance along the glenoacetabular axis to LB was defined as the “COM forelimb bias,” representing the percentage of weight supported by forelimbs in Batrachotomus (see Henderson, 2003; Willey et al., 2004). COM forelimb bias for dogs was obtained from Griffin et al. (2004).
TABLE 1. Body parameters used as inputs to the models.
Trackway Data
The ichnotaxa Isochirotherium and Brachychirotherium are generally attributed to large pseudosuchian trackmakers, potentially “rauisuchians” like Batrachotomus or related species (Diedrich, 2015; Apesteguía et al., 2021; Hminna et al., 2021; Klein and Lucas, 2021). Trackway data of interest include stride length, pes and manus length, and ipsilateral pes-to-manus distance within a footprint “set.” For each trackway, we compiled all available data. For example, all pes lengths were used to calculate mean pes length, even if some were not associated with a manus. Data were collected from published sources. While these sources sometimes assigned trackways to ichnospecies, for this analysis, we grouped ichnotaxa at the ichnogenus level.
Data from Tables
Published table data were used when available (Petti et al., 2009; Diedrich, 2012; Diedrich, 2015). Manus-pes distance was consistently reported as inter-print distance (IPD). In these cases, midpoint-midpoint distance was calculated as (Pes Length+Manus Length)/2 +IPD.
Petti et al. (2009) assigned four trackways to Brachychirotherium (BsZ-A, -D, -E, and -F). Only BsZ-A and BsZ-D included manus-pes distance and were included in this study. Diedrich (2012) reported three Isochirotherium trackways in Table 1, and the values were interpreted as means. No variational statistics were provided, except for the number of manus/pes sets. Diedrich (2015) reported a single Isochirotherium trackway in their Table 1, with data for each manus/pes set. Stride lengths were reported for every third manus/pes set in this table.
Data from Figures
Additional trackway data were extracted from published orthogonal-view photographs or trackway traces using ImageJ (Abramoff et al., 2004). Generally, for each pes print, the tip of the third digit and the caudal-most point of the fifth digit were digitized, with the craniocaudal axis defined along the third digit’s axis (Figure 1). If digit five was missing, the caudal-most point of the metatarsal pad was used. This process was repeated for manus tracks, except for Brachychirotherium. Due to manus rotation in this ichnogenus, the second digit was used for the craniocaudal axis, and the tip was digitized accordingly.
Midpoints for each manus or pes were calculated, and the stride vector was defined as the displacement between midpoints of successive pes footfalls on the same side (D→). The midpoint was chosen to represent the average center of pressure for footfalls and reduce reliance on precise point measurements. The manus-pes midpoint vector is the displacement from pes to manus midpoint (d→). Manus-pes distance was calculated as the projection of this vector onto the average stride vector for the trackway (d→⋅D→av/||D→av||).
Clark and Corrance (2009) presented photos of single manus-pes sets of Isochirotherium. These were digitized as described above. The set from their Figure 7B corresponded to trackway BWF_5 data in Table 1 of Clark et al. (2002), initially assigned to Chirotherium. The trackway set from Figure 6C of Clark and Corrance (2009) corresponded to trackway SLID_1 data in their Table 1. However, the digitized pes length in this case (0.13 m) was much smaller than the smallest reported value in their Table 1 (0.21 m). Based on the trackway photo (Figure 6 in Clark et al., 2002), the pes was the sixth print of SLID_1 in their Table 1, and the caudal portion might have been cropped from Figure 6C of Clark and Corrance (2009). To correct this, the caudal point was extended by the length of pes 6 from their Table 1 (0.28 cm). As no orthogonal views were available for either trackway, projection to stride was not possible, and the absolute pes-to-manus midpoint distance was used. Pes length data for the final print in BWF_5 were excluded due to poor preservation.
Apesteguía et al. (2021) assigned four trackways to Brachychirotherium, R1, R2-t1, -t5, and -t9, presented in Figures 4A, 5B of their publication. Trackway R1 included a trailing left manus without an associated pes. A manus-pes midpoint distance to the next left pes was calculated and subtracted from the stride length to obtain the pes-manus midpoint distance. The same left pes was used to calculate the pes-manus midpoint distance for the subsequent left manus print. Trackway R2-t1 appeared to have a slight turn at the beginning, evident in manus-pes sets 1 and 2. The rest of the trackway appeared straight. Therefore, the first two manus-pes sets were excluded for track phase and stride length calculations but included for trackmaker size. Prints in trackway R2-t5 were relatively poorly preserved but sufficient for ichnotaxon identification (Brachychirotherium). For this trackway, cranial-most and caudal-most points of each print were digitized, with craniocaudal defined by the trackway direction where digits were indistinguishable.
Klein et al. (2006) reported a single Brachychirotherium trackway in their Figure 7. As digits of the first manus were indistinguishable, the cranial-most portion of the print along the trackway direction was used.
Hminna et al. (2021) reported a single Brachychirotherium trackway in their Figure 4. Only one set of well-preserved sequential ipsilateral pes footprints was present in this trackway. Therefore, sequential ipsilateral manus footprints were used to measure stride length (total 4 in the trackway).
Calculation of Gait Parameters
Trackmaker hip height was estimated as mean track pes length multiplied by the hip height-to-pes length ratio from the Batrachotomus model. Non-dimensional speed (UH′=U/gH) was estimated from non-dimensional stride length (DH′=D/H, where D is stride length) using Alexander’s (1976) dynamic similarity relation:
UH′=0.25DH′1.67.(2)
Trackway stride length and speed were model inputs, while track phase was compared to optimization predictions. An analytical approximation (Stevens et al., 2016) for track phase (ϕT) as a function of ipsilateral limb phase (ϕL), with LBx being horizontal glenoacetabular distance, was used:
ΦT=mod(ΦL+LBx/D,1).(3)
This was also compared to trackway and optimization results. LBx is derived from LB, the absolute glenoacetabular distance, as LBx=LB2−(LH−LF)2 in standing (Figure 2).
LBx can be directly estimated from trackways if periods of quadruple stance are assumed (when all limbs are simultaneously in contact). For a trot, this parameter is the distance from the midpoint between left and right pes prints to the midpoint between subsequent left and right manus prints. For this study, this is equivalent to:
LBx=d+D/2.(4)
See Supplementary Figure S1 for geometric proof. While Stevens et al. (2016) demonstrated potential ambiguities for small stride lengths, the stride lengths in this study are sufficiently large to avoid ambiguity regarding simultaneous contact prints (assuming quadrupedal stance).
Results
Belgian Malinois Dogs
For dogs, optimization predicts a lateral sequence gait with limb phase ϕL=0.25 transitioning to a trot with ϕL=0.5, consistent with observed dog behavior (Figure 3). The predicted track phase falls within natural variation (2 standard deviations, from Maes et al., 2008) and captures the trend of track phase change with speed, although it is consistently lower than the mean empirical value.
FIGURE 3. Dog track phase decreases with speed, showing a jump at the walk-trot transition. Lines of best fit to empirical data are from Maes et al. (2008), with filled areas representing two standard deviations from the mean. Black solid and dotted lines indicate ϕL=0.25 and 0.5, respectively.
Varying the force-rate penalty c has minimal qualitative impact on optimization results, except for the walk-trot transition point. The natural walk-trot transition occurs around UH′=0.8. The simulated transition point shifts from about UH′=0.7 at the highest force-rate penalty (c′=3×10−3) to UH′=0.8 at the lowest (c′=3×10−5). Polet and Bertram (2019) found c′=3×10−3 best matched dog duty factors and ground reaction force profiles at an intermediate walking speed. Ground reaction forces at this force-rate penalty (Figure 4) qualitatively match the double-humped profile of walking (Jayes and Alexander, 1978) and the single-humped profile of trotting (Bertram et al., 2000) in dogs.
FIGURE 4. Ground reaction forces from predictive simulation at a walking speed (A) and trotting speed (B) in a dog model with a force-rate penalty c′ of 3×10−3. The simulation correctly predicts double-humped walking profiles at slow speeds and single-humped trotting profiles at faster speeds, despite allowing any symmetrical profile.
Trackway Data
Trackways exhibited variations in size and length, with mean pes lengths in Isochirotherium ranging from 0.22 to 0.34 m and in Brachychirotherium from 0.12 to 0.39 m (Table 2). Isochirotherium normalized speed (estimated via Eq. 2) ranged from 0.40 to 0.79, typical of moderate to fast walking or slow running. Brachychirotherium speeds varied more widely, from slow walking at 0.31 to fast running at 1.92. The mean manus-to-pes length ratio across all trackways was 0.35 ± 0.05 for Isochirotherium and 0.38 ± 0.09 for Brachychirotherium (±standard deviation).
TABLE 2. Summary data for Isochirotherium and Brachychirotherium trackways. Values are means ± standard deviation (sample size). Speed is normalized to presumed hip height. ET, Early Triassic; MT, Middle Triassic; LT, Late Triassic; A, Anisian; C, Carnian; N, Norian; S, “Scythian.”
Overall, fossil trackways show a sharp decrease in track phase with increasing speed at slower speeds, following the analytical line for a trot (Figure 5). Above UH′=0.6, the track phase remains constant at approximately 0.15 until UH′=1, where it sharply drops to around 0.10.
FIGURE 5. Track phase plotted against speed for Batrachotomus versus fossil chirotheriid trackways. Different levels of force-rate penalty c′ are indicated by different symbols. The lowest force-rate penalty of 3×10−5 best fits trackway data. Both simulation and trackway data show a gradual reduction in track phase at slow walking, corresponding to a trot based on the analytical curve (dashed line). This abruptly changes to a constant track phase with increasing speed, reflecting the increased limb phase according to analytical curves. Near UH′=1, the theoretical upper limit for pendular walking, both simulation and trackway data exhibit a sharp reduction in track phase, shifting toward a diagonal sequence singlefoot walk (ϕL=0.75). At intermediate speeds, the simulation consistently overestimates the trackway phase. Silhouette of Batrachotomus by Scott Hartman, used under a CC BY 3.0 license.
Horizontal glenoacetabular distance (LBx) was estimated from trackways using Eq. (4) for estimated non-dimensional speeds below 0.4 (where quadruple limb stance might have occurred). Only two trackways met this criterion, both Brachychirotherium (Apesteguía et al., 2021, trackways R1 and R2-t5), yielding LBx/LP estimates of 3.11 and 2.95, respectively (where LP is mean pes length). The reconstructed Batrachotomus model has LBx/LP=2.85 and LB/LP= 3.08.
Scaling fossil trackways to the Batrachotomus reconstruction yields estimated LBx values of 0.81 and 0.79 m. The reconstruction itself gives LBx = 0.74 m and LB=0.80 m.
Batrachotomus Simulation Results
Optimization predicts that Batrachotomus would have used a walking trot at slow speeds (UH′≤0.4), transitioning to a diagonal sequence gait at faster speeds (Figure 5). The walking trot is characterized by a sharp decrease in track phase with increasing speed. With relatively high force-rate penalties, the transition to a diagonal sequence, singlefoot gait corresponds to a discontinuity in track phase around UH′=0.4. For the lowest force-rate penalty (c′= 3×10−5), the track phase jumps to a plateau, remaining constant at about 0.33 while the limb phase gradually increases. As the limb phase approaches a diagonal sequence in singlefoot (ϕL=0.75), the track phase decreases sharply again with increasing speed, maintaining a roughly constant limb phase.
At the highest force-rate penalty, the walking trot shows typical “vaulting” double-humped ground reaction force profiles (Figure 6A), with simultaneous fore- and hindlimb contacts. As speed increases, hindlimbs continue to vault, while forelimb force profiles shift to single-humped, indicating a bouncing mode (Figure 6B). At the theoretical vaulting limit for hindlimbs (UH′=1), hindlimbs also transition to bouncing (Figure 6C) (see Supplementary Video S1 for animations). This general gait shift is also seen at the lowest force-rate penalty (Figures 6D–F), although peak forces approach impulsivity, as expected from work-minimizing optimization (Srinivasan, 2010). At walking speeds, solutions may show periods during stance where hindlimbs are completely offloaded (Figure 6D).
FIGURE 6. Predicted ground reaction forces from simulation for the unconstrained Batrachotomus model with force-rate penalties of 3×10−3 (upper row) and 3×10−5 (lower row). (A) At slow speeds, a walking trot pattern is observed, with double-humped profiles typical of vaulting. (B) At intermediate speeds, forelimbs transition to a single-humped, bouncing mode. (C) At the fastest speeds, hindlimbs also transition to bouncing. (D–F) Similar behavior is also seen at a higher force-rate penalty, but unrealistic peak forces and midstance force unloading at some speeds (D) make this force-rate penalty less plausible (see Supplementary Video S1 for animations).
When solutions are constrained to match fossil trackway track phase trends, ground reaction forces differ somewhat from the unconstrained case. Before transitioning to a constant track phase, the optimal solution shifts from a vaulting walking trot to vaulting hindlimbs with skewed forelimb ground reaction forces, indicating an asymmetrical (generative) bouncing mode (Figure 7A, see Supplementary Video S2). This gait persists at the transition to constant track phase (Figure 7B) but gradually shifts to bouncing in both fore- and hindlimbs, similar to the unconstrained case (Figure 7C). As speed increases further, the solution remains similar but with lower duty factor and higher peak forces.
FIGURE 7. Predicted ground reaction forces for the Batrachotomus model constrained to match the empirical track phase. (A) By a speed of 0.49, the optimal solution involves skewed ground reaction forces in forelimbs and semi-vaulting forces in hindlimbs. (B) This pattern is maintained at the transition point where track phase remains constant with increasing speed. (C) By a speed of 0.80, the solution shifts to a running tölt, similar to the unconstrained case but with different forelimb contact timing. The same solution is found at faster speeds in the constrained case, but with lower duty factor and higher peak forces (see Supplementary Video S2 for animations).
Constrained solutions have higher costs than unconstrained solutions (Supplementary Figure S2A). The cost difference is greatest at intermediate speeds and diminishes at higher speeds. At UH′=0.55, the cost of transport for the constrained case is 1.2 times that of the unconstrained case (Supplementary Figure S2B). At UH′=1.92, this ratio reduces to 1.01.
Discussion
The simulation method accurately predicts canine track phase within natural variation, though predicted values are consistently below mean empirical values (Figure 3). The model correctly predicts symmetrical walking and running gaits in dogs, their ground reaction force profiles (Figure 4), and gait transition speed. This strengthens confidence in applying the model to organisms where only stride length, size, and shape can be estimated, and gait is unknown, allowing for valuable comparisons between archosaur and crocodile locomotion.
Brachychirotherium trackways exhibit a consistent track phase-speed pattern: a sharp decrease with increasing speed below UH′=0.6, followed by a constant track phase up to UH′=1, and then a sudden drop (Figure 5). Isochirotherium trackways also follow this pattern, but data are absent above dimensionless speeds of 0.8. This broad similarity in track phase pattern with estimated speed supports combining these ichnogenera for analysis.
Across all force-rate penalties, simulations correctly predict a walking trot at slow speeds, closely following the analytical curve. In all cases, the optimal gait transitions around UH′=0.4, shifting to a higher track phase. This indicates not only an increase in limb phase from trot to diagonal sequence gait but also a fundamental gait type shift. While slow-speed gait ground reaction force profiles show the double-humped shape characteristic of vaulting (Figures 6A,D), the faster gait is a hybrid, with vaulting hindlimbs and bouncing forelimbs (Figures 6B,E). This closely resembles the slow tölt of Icelandic horses (Biknevicius et al., 2004). Like Icelandic horses, simulations switch to a “running tölt” at higher speeds: a four-beat symmetrical gait with single-humped ground reaction force profiles in all limbs (Figure 6C). The tölt is a rare gait in mammals (Vincelette, 2021) and unprecedented in archosaurs, although detected in fossil horse trackways (Renders, 1984; Vincelette, 2021).
However, at speeds above 0.4, simulations diverge significantly from fossil trackways in track phase. Nonetheless, trackways show distinct track phase changes around UH′=0.6 and 1.0, coinciding with gait changes in simulations. When simulations are constrained to match fossil trackway track phase, solutions remain qualitatively similar to the unconstrained case, showing bouncing forelimbs and vaulting hindlimbs at UH′=0.6 (Figure 7)—similar to a slow tölt—before transitioning to bouncing in both limbs at UH′=1—similar to a fast tölt. The sharp changes in fossil track phase at UH′=0.6 and 1.0, their correspondence with limb phase changes based on analytical relations, and multiple simulations predicting gait transitions near these speeds, suggest that fossil data indicate a gait transition in pseudosuchian trackmakers.
Although fossil trackways show manus placed cranially to the ipsilateral pes in a couplet, qualitatively similar to modern crocodiles in a walking trot (Kubo, 2008), long trackway stride lengths imply gaits more akin to a dissociated trot or diagonal sequence. Modern crocodiles, the closest living relatives of Batrachotomus, typically do not transition to a four-beat gait at higher speeds, instead continuing to trot or switching to asymmetrical gaits (Hutchinson et al., 2019). While extant crocodiles do exhibit increased limb phase with speed for symmetrical gaits (Supplementary Figure S3), the change is more gradual than observed here. Crocodiles do not consistently walk with the lateral sequence diagonal-couplet gait (Supplementary Figure S3; Hutchinson et al., 2019) considered ancestral for quadrupedal gnathostomes (Wimberly et al., 2021). These results provide the first evidence that extinct pseudosuchians exhibited gaits different from modern relatives and the first evidence of a gait transition in an extinct pseudosuchian, highlighting a divergence in locomotor evolution between archosaurs and crocodiles.
The use of a two-beat gait at slow speeds and a four-beat gait at faster speeds aligns with Polet’s (2021b) analysis. The large pitch moment of inertia of Batrachotomus relative to glenoacetabular distance gives it a Murphy number of 3. Above a Murphy number of 1, a four-beat run becomes optimal because pitching the body is energetically cheaper than vertical body movement. Thus, rejecting pitching via a trotting gait is less economical. However, maintaining vaulting in a typical four-beat walk requires body pitching, energetically expensive for animals with large pitch moment of inertia. This favors a walking trot at slow speeds in archosaurs compared to crocodiles, which may have different inertial properties.
Both unconstrained and constrained cases show similar ground reaction force solutions, but constrained cases are skewed. In unconstrained cases, the forelimb is nearly vertical during hindlimb double support (Figure 8A). Peak force at midstance effectively offloads body weight during the costly hindlimb step transition (Schroeder and Bertram, 2018). Simultaneously, the symmetrical, single-hump profile acts as a distributed pseudo-elastic “collision” (Figure 6B), minimizing limb work and managing force-rate penalty (Ruina et al., 2005; Rebula and Kuo, 2015).
FIGURE 8. Geometry at hindlimb step transition for unconstrained and constrained track phase cases, both at UH′=0.49 and c′=0.003. (A) In the unconstrained case, the forelimb is nearly vertical, effectively offloading hindlimbs. Resulting ground reaction forces (GRF) are symmetrical, as shown in Figure 6B. (B) In the constrained track phase case, the shoulder is extended, providing both forward and vertical propulsive force, assisting hindlimb pushoff. GRF forces become asymmetrical, as shown in Figure 7A.
In constrained cases, the shoulder is flexed during double support (Figure 8A), meaning leg pushing forces generate both vertical and forward COM propulsion. This is less effective for weight offloading during support transfer but aids hindlimb pre-transfer pushoff, mitigating work-related energetic losses (Ruina et al., 2005; Rebula and Kuo, 2015; Schroeder and Bertram, 2018). Consequently, forelimb force can increase, while the second hindlimb peak can decrease (Figure 7A). However, asymmetry results in a generative (plastic) distributed “collision,” typically requiring more work than a symmetrical (pseudo-elastic) collision (Ruina et al., 2005).
Trackway transition away from the analytical walking trot line occurs around UH′=0.6 (Figure 5), below the pendular walk maximum speed of UH′=1 (Usherwood, 2005) but matching the four-beat gait transition predicted by Polet (2021b) for a Murphy number of 3 in a perfectly symmetrical model.
When gait transition appears in trackway data, ipsilateral manus and pes prints are nearly overstepping. Further speed increase without gait change would cause overstepping and ipsilateral manus-pes collision unless limb orientations changed. Is the inferred gait transition forced by this physical constraint, rather than energetic considerations?
Modern crocodiles avoid foot collision in walking trots by changing body yaw, misaligning the craniocaudal axis with motion direction, placing manus prints slightly left or right of pes prints (Kubo, 2008; Figures 2A,E therein). Dogs also employ this strategy when trotting (Murie and Elbroch, 2005). There is no a priori reason to believe that “rauisuchians” like Batrachotomus couldn’t yaw their bodies similarly to continue walking trots at higher speeds. Likewise, the planar model used here is not constrained to prevent ipsilateral leg/foot collisions. The transition, in this case, is purely driven by energetic factors.
The earlier trot transition in simulations (around UH′= 0.40) seems driven by the relatively short forelimbs of Batrachotomus (61% of hindlimb length). A 10% increase in model forelimb length increases transition speed from 0.40 to 0.45, and a 50% increase changes it to 0.49 (Supplementary Figures S4, 5), simultaneously decreasing track phase value at transition speed. This may reflect morphological differences between real trackmakers and Batrachotomus. Reconstruction shows a manus-to-pes length ratio of 0.58, compared to 0.35–0.38 in trackways. This aligns with fossil footprint morphology, showing digitigrade or semi-digitigrade manus prints in forelimbs (Diedrich, 2015; Klein and Lucas, 2021), similar to modern crocodiles (Hutson and Hutson, 2015), whereas Batrachotomus is interpreted as plantigrade. It is also possible that Batrachotomus in life had a less flexed elbow than interpreted here, effectively increasing forelimb length (Figure 2).
This is also reflected in the horizontal glenoacetabular distance-to-pes length ratio (LBx/LP) in Brachychirotherium trackways (2.95–3.11), which more closely matches the Batrachotomus reconstruction when absolute glenoacetabular distance is used (LB/LP= 3.08) rather than LBx/LP (2.85). This could result from more equal glenoid and acetabulum height than assumed, but also from morphological differences between Batrachotomus and Brachychirotherium, or uncertainties in the Batrachotomus pedal reconstruction used.
While simulations provide evidence for gait transition in trackmakers, they do not accurately predict track phase except at slow speeds. Discrepancies may arise from model inaccuracies in capturing “rauisuchian” gait energetics due to neglected morphological features (e.g., leg inertia) or physiological characteristics (e.g., muscle force-velocity properties). Trackmakers may also not follow Alexander’s (1976) stride length-speed relationship (primarily based on mammals), possibly due to reduced hip flexion-extension in some “rauisuchians” (Nesbitt et al., 2013), not considered in this analysis. Furthermore, trackmakers may differ from Batrachotomus in proportions, mass distribution, or other key aspects. Finally, energetics might not be the primary determinant of locomotion for these trackmakers; soft substrate track formation could influence gait choice and phase relationships. Future pseudosuchian locomotion simulation developments can address these issues by increasing realism and evaluating models in extant crocodiles, providing a stronger basis for archosaur compared to crocodile locomotion.
Conclusion
We applied a planar, generalized quadrupedal model to the gait of Batrachotomus kupferzellensis, an extinct crocodile-line (pseudosuchian) archosaur. We compared predictions to fossil trackways potentially left by Batrachotomus relatives. When optimized to minimize leg work and force rate squared, the model correctly predicted a sharp track phase reduction with speed at low speeds, corresponding to a trot. Subsequently, the model predicted a transition to a four-beat walk similar to a slow tölt, near where fossil trackways deviated from the walking trot trajectory and appeared to transition to a diagonal sequence gait. Finally, when fossil trackways showed another sharp track phase transition, the model predicted a transition to a four-beat gait similar to a fast tölt. This is the first evidence of gait transition in an extinct pseudosuchian and that “rauisuchians” like Batrachotomus may have exhibited gaits different from modern crocodiles, expanding our understanding of archosaur locomotion compared to crocodiles.
Given Batrachotomus‘s inferred features shared with both modern crocodiles and mammals, trajectory optimization offers a unique opportunity to understand gait in the absence of direct analogues. Optimization results suggest that Batrachotomus‘s large pitch moment of inertia and erect limb posture favored a tölt-like gait, unseen in any extant archosaurian group and rare in mammals. This raises intriguing questions about the evolution and frequency of this gait suite in pseudosuchians and the ancestral state for Archosauria (birds, crocodiles, and all extinct descendants of their common ancestor, including Mesozoic dinosaurs). More sophisticated three-dimensional models incorporating lateral motions and realistic morphology (e.g., Bishop et al., 2021) or neuromuscular control and stability analyses may provide further insights into archosaur locomotion compared to crocodiles.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
The project was conceived by DP and JH, and both authors gathered data from the literature and wrote the manuscript. DP digitized trackways, analyzed data, and performed the simulations.
Funding
This work was supported by the European Research Council Advanced Investigator Award (Grant agreement ID: 695517) to JH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Ashleigh Wiseman for her prior helpful discussions. We also thank Vivian Allen, Andrew Cuff and Peter Bishop for preparing the Batrachotomus model, as well as Eudald Mujal and Shinya Aoi for their thoughtful reviews, which greatly enhanced the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.800311/full#supplementary-material
References
Abramoff, M. D., Magalhães, P. J., and Ram, S. J. (2004). Image Processing with ImageJ. Biophotonics Int. 11, 36–42.
Alexander, R. M. (1976). Estimates of Speeds of Dinosaurs. Nature 261, 129–130. doi:10.1038/261129a0
Apesteguía, S., Riguetti, F., Citton, P., Veiga, G. D., Poiré, D. G., de Valais, S., et al. (2021). The Ruditayoj-Tunasniyoj Fossil Area (Chuquisaca, Bolivia): a Triassic Chirotheriid Megatracksite and Reinterpretation of Purported Thyreophoran Tracks. Hist. Biol. 33, 2883–2896. doi:10.1080/08912963.2020.1833328
Bertram, J. E. A., Lee, D. V., Case, H. N., and Todhunter, R. J. (2000). Comparison of the Trotting Gaits of Labrador Retrievers and Greyhounds. Am. J. Vet. Res. 61, 832–838. doi:10.2460/ajvr.2000.61.832
Biknevicius, A. R., Mullineaux, D. R., and Clayton, H. M. (2004). Ground Reaction Forces and Limb Function in Tölting Icelandic Horses. Equine Vet. J. 36, 743–747. doi:10.2746/0425164044848190
Bishop, P. J., Bates, K. T., Allen, V. R., Henderson, D. M., Randau, M., and Hutchinson, J. R. (2020). Relationships of Mass Properties and Body Proportions to Locomotor Habit in Terrestrial Archosauria. Paleobiology 46, 550–568. doi:10.1017/pab.2020.47
Bishop, P. J., Falisse, A., De Groote, F., and Hutchinson, J. R. (2021). Predictive Simulations of Running Gait Reveal a Critical Dynamic Role for the Tail in Bipedal Dinosaur Locomotion. Sci. Adv. 7, eabi7348. doi:10.1126/sciadv.abi7348
Bonaparte, J. F. (1984). Locomotion in Rauisuchid Thecodonts. J. Vertebr. Paleontol. 3, 210–218. doi:10.1080/02724634.1984.10011976
Clark, N. D. L., Aspen, P., and Corrance, H. (2002). Chirotherium barthii Kaup 1835 from the Triassic of the Isle of Arran, Scotland. Scottish J. Geology. 38, 83–92. doi:10.1144/sjg38020083
Clark, N. D. L., and Corrance, H. (2009). New Discoveries of Isochirotherium herculis (Egerton 1838) and a Reassessment of Chirotheriid Footprints from the Triassic of the Isle of Arran, Scotland. Scottish J. Geology. 45, 69–82. doi:10.1144/0036-9276/01-362
Diedrich, C. (2015). Isochirotherium Trackways, Their Possible Trackmakers (?Arizonasaurus): Intercontinental Giant Archosaur Migrations in the Middle Triassic Tsunami-Influenced Carbonate Intertidal Mud Flats of the European Germanic Basin. Carbonates Evaporites 30, 229–252. doi:10.1007/s13146-014-0228-z
Diedrich, C. (2012). Middle Triassic Chirotherid Trackways on Earthquake Influenced Intertidal Limulid Reproduction Flats of the European Germanic Basin Coasts. Cent. Eur. J. Geo. 4, 495–529. doi:10.2478/s13533-011-0080-9
Gill, P. E., Murray, W., and Saunders, M. A. (2005). SNOPT: An SQP Algorithm for Large-Scale Constrained Optimization. SIAM Rev. 47, 99–131. doi:10.1137/s0036144504446096
Gill, P. E., Murray, W., Saunders, M. A., and Wong, E. (2015). User’s Guide for SNOPT 7.5: Software for Large-Scale Nonlinear Programming. La Jolla, CASan Diego: Department of Mathematics, University of California.
Gower, D. J., and Schoch, R. R. (2009). Postcranial Anatomy of the Rauisuchian archosaur Batrachotomus kupferzellensis. J. Vertebr. Paleontol. 29, 103–122. doi:10.1080/02724634.2009.10010365
Gower, D. (1999). The Cranial and Mandibular Osteology of a New Rauisuchian Archosaur from the Middle Triassic of Southern Germany. Stuttgarter Beiträge Zur Naturkunde. Serie B (Geologie Paläontologie) 280, 1–49.
Griffin, T. M., Main, R. P., and Farley, C. T. (2004). Biomechanics of Quadrupedal Walking: How Do Four-Legged Animals Achieve Inverted Pendulum-like Movements? J. Exp. Biol. 207 (2072), 3545–3558. doi:10.1242/jeb.01177
Henderson, D. M. (2003). Effects of Stomach Stones on the Buoyancy and Equilibrium of a Floating Crocodilian: a Computational Analysis. Can. J. Zool. 81, 1346–1357. doi:10.1139/z03-122
Hminna, A., Klein, H., Zouheir, T., Lagnaoui, A., Saber, H., Lallensack, J. N., et al. (2021). The Late Triassic Archosaur Ichnogenus Brachychirotherium: First Complete Step Cycles from Morocco, North Africa, with Implications for Trackmaker Identification and Ichnotaxonomy. Hist. Biol. 33, 723–736. doi:10.1080/08912963.2019.1658097
Hutchinson, J. R., Felkler, D., Houston, K., Chang, Y.-M., Brueggen, J., Kledzik, D., et al. (2019). Divergent Evolution of Terrestrial Locomotor Abilities in Extant Crocodylia. Sci. Rep. 9, 19302. doi:10.1038/s41598-019-55768-6
Hutson, J. D., and Hutson, K. N. (2015). Inferring the Prevalence and Function of finger Hyperextension in Archosauria from finger‐joint Range of Motion in the American alligator. J. Zool 296, 189–199. doi:10.1111/jzo.12232
Jayes, A. S., and Alexander, R. M. (1978). Mechanics of Locomotion of Dogs (Canis familiaris) and Sheep (Ovis aries). J. Zoolog. 185, 289–308. doi:10.1111/j.1469-7998.1978.tb03334.x
Klein, H., Lucas, S. G., and Haubold, H. (2006). “Tetrapod Track Assemblage of the Redonda Formation (Upper Triassic, Chinle Group) in East-central New Mexico – Reevaluation of Ichnofaunal Diversity from Studies of New Material,” in The Triassic-Jurassic Terrestrial Transition. Editors J. D. Harris, S. G. Lucas, J. A. Speilmann, M. G. Lockley, A. R. C. Milner, and J. I. Kirkland (Albuquerque, New Mexico: New Mexico Museum of Natural History and Science), 241–250.
Klein, H., and Lucas, S. G. (2021). The Triassic Tetrapod Footprint Record. Albuquerque, New Mexico: New Mexico Museum of Natural History and Science.
Kubo, T. (2008). In Quest of the Pteraichnus Trackmaker: Comparisons to Modern Crocodilians. Acta Palaeontologica Pol. 53, 405–412. doi:10.4202/app.2008.0304
Maes, L. D., Herbin, M., Hackert, R., Bels, V. L., and Abourachid, A. (2008). Steady Locomotion in Dogs: Temporal and Associated Spatial Coordination Patterns and the Effect of Speed. J. Exp. Biol. 211, 138–149. doi:10.1242/jeb.008243
Murie, O. J., and Elbroch, M. (2005). A Field Guide to Animal Tracks. New York, NY: Houghton Mifflin Harcourt.
Nesbitt, S. J., Brusatte, S. L., Desojo, J. B., Liparini, A., De França, M. A. G., Weinbaum, J. C., et al. (2013). Rauisuchia. Geol. Soc. Lond. Spec. Publications 379 (1), 241–274. doi:10.1144/SP379.1
Nyakatura, J. A., Melo, K., Horvat, T., Karakasiliotis, K., Allen, V. R., Andikfar, A., et al. (2019). Reverse-engineering the Locomotion of a Stem Amniote. Nature 565, 351–355. doi:10.1038/s41586-018-0851-2
Parrish, J. M. (1986). Locomotor Adaptations in the Hindlimb and Pelvis of the Thecodontia. Hunteria 1, 2–35.
Patterson, M. A., and Rao, A. V. (2014). GPOPS-II. ACM Trans. Math. Softw. 41, 1–37. doi:10.1145/2558904
Petti, F. M., Avanzini, M., Nicosia, U., Girardi, S., Bernardi, M., Ferretti, P., et al. (2009). Late Triassic (Early-middle Carnian) Chirotherian Tracks from the Val Sabbia Sandstone (Eastern Lombardy, Brescian Prealps, Northern Italy). Riv. Ital. di Paleontol. e Stratigr. 115, 277–290. doi:10.13130/2039-4942/6384
Polet, D. T., and Bertram, J. E. A. (2019). An Inelastic Quadrupedal Model Discovers Four-Beat Walking, Two-Beat Running, and Pseudo-elastic Actuation as Energetically Optimal. PLOS Comput. Biol. 15, e1007444. doi:10.1371/journal.pcbi.1007444
Polet, D. T. (2021a). Delyle/Optimize-Symmetrical-Quadruped: Optimize Symmetrical Quadruped. Zenodo. doi:10.5281/zenodo.5593594
Polet, D. T. (2021b). The Murphy Number: How Pitch Moment of Inertia Dictates Quadrupedal Walking and Running Energetics. J. Exp. Biol. 224, jeb228296. doi:10.1242/jeb.228296
Rebula, J. R., and Kuo, A. D. (2015). The Cost of Leg Forces in Bipedal Locomotion: A Simple Optimization Study. PLOS ONE 10, e0117384. doi:10.1371/journal.pone.0117384
Renders, E. (1984). The Gait of Hipparion Sp. From Fossil Footprints in Laetoli, Tanzania. Nature 308, 179–181. doi:10.1038/308179a0
Ruina, A., Bertram, J. E. A., and Srinivasan, M. (2005). A Collisional Model of the Energetic Cost of Support Work Qualitatively Explains Leg Sequencing in Walking and Galloping, Pseudo-elastic Leg Behavior in Running and the Walk-To-Run Transition. J. Theor. Biol. 237, 170–192. doi:10.1016/j.jtbi.2005.04.004
Schroeder, R. T., and Bertram, J. E. (2018). Minimally Actuated Walking: Identifying Core Challenges to Economical Legged Locomotion Reveals Novel Solutions. Front. Robot. AI 5, 58. doi:10.3389/frobt.2018.00058
Srinivasan, M. (2010). Fifteen Observations on the Structure of Energy-Minimizing Gaits in many Simple Biped Models. J. R. Soc. Interf. 8, 74–98. doi:10.1098/rsif.2009.0544
Stevens, K. A., Ernst, S., and Marty, D. (2016). “Uncertainty and Ambiguity in the Interpretation of Sauropod Trackways,” in Dinosaur Tracks: The Next Steps Life of the Past. Editors P. L. Falkingham, D. Marty, and A. Richter (Bloomington, IN: Indiana University Press), 227–243.
Usherwood, J. R. (2020). An Extension to the Collisional Model of the Energetic Cost of Support Qualitatively Explains Trotting and the Trot-Canter Transition. J. Exp. Zool. 333, 9–19. doi:10.1002/jez.2268
Usherwood, J. R. (2005). Why Not Walk Faster? Biol. Lett. 1, 338–341. doi:10.1098/rsbl.2005.0312
Vincelette, A. (2021). Determining the Gait of Miocene, Pliocene, and Pleistocene Horses from Fossilized Trackways. Foss. Rec. 24, 151–169. doi:10.5194/fr-24-151-2021
Wikimedia Commons (2010). Diagram of Brachychirotherium. Available at: https://commons.wikimedia.org/wiki/File:Brachychirotherium.svg (Accessed September 9, 2021).
Willey, J. S., Biknevicius, A. R., Reilly, S. M., and Earls, K. D. (2004). The Tale of the Tail: Limb Function and Locomotor Mechanics in Alligator mississippiensis. J. Exp. Biol. 207, 553–563. doi:10.1242/jeb.00774
Wimberly, A. N., Slater, G. J., and Granatosky, M. C. (2021). Evolutionary History of Quadrupedal Walking Gaits Shows Mammalian Release from Locomotor Constraint. Proc. R. Soc. B. 288, 20210937. doi:10.1098/rspb.2021.0937
Xi, W., Yesilevskiy, Y., and Remy, C. D. (2016). Selecting Gaits for Economical Locomotion of Legged Robots. Int. J. Robotics Res. 35, 1140–1154. doi:10.1177/0278364915612572
Yesilevskiy, Y., Yang, W., and Remy, C. D. (2018). Spine Morphology and Energetics: How Principles from Nature Apply to Robotics. Bioinspir. Biomim. 13, 036002. doi:10.1088/1748-3190/aaaa9e
Keywords: locomotion, predictive simulation, Pseudosuchia, fossil trackways, energetics, Chirotheriidae
Citation: Polet DT and Hutchinson JR (2022) Estimating Gaits of an Ancient Crocodile-Line Archosaur Through Trajectory Optimization, With Comparison to Fossil Trackways. Front. Bioeng. Biotechnol. 9:800311. doi: 10.3389/fbioe.2021.800311
Received: 22 October 2021; Accepted: 30 December 2021;Published: 03 February 2022.
Edited by:
Bernardo Innocenti, Université libre de Bruxelles, Belgium
Reviewed by:
Eudald Mujal, Staatliches Museum für Naturkunde Stuttgart, Germany
Shinya Aoi, Kyoto University, Japan
Copyright © 2022 Polet and Hutchinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delyle T. Polet, ZHBvbGV0QHJ2Yy5hYy51aw==; John R. Hutchinson, amh1dGNoaW5zb25AcnZjLmFjLnVr
†ORCID: Delyle T. Polet, orcid.org/0000-0002-8299-3434; John R. Hutchinson, orcid.org/0000-0002-6767-7038