A Student Compares The Boiling Point Of Substances by assessing intermolecular forces, molecular weight, and molecular symmetry. At COMPARE.EDU.VN, we offer detailed comparisons to help you understand these complex relationships and make informed decisions. Discover how molecular properties affect boiling points with our comprehensive guides, enhancing your understanding of physical chemistry.
1. Understanding Boiling Points: Intermolecular Forces
Boiling points are a crucial physical property, reflecting the strength of the forces holding molecules together. A higher boiling point indicates stronger intermolecular attractions, requiring more energy to transition a substance from liquid to gas. COMPARE.EDU.VN offers tools to compare these forces across different substances, aiding students and professionals alike.
1.1 The Role of Intermolecular Forces
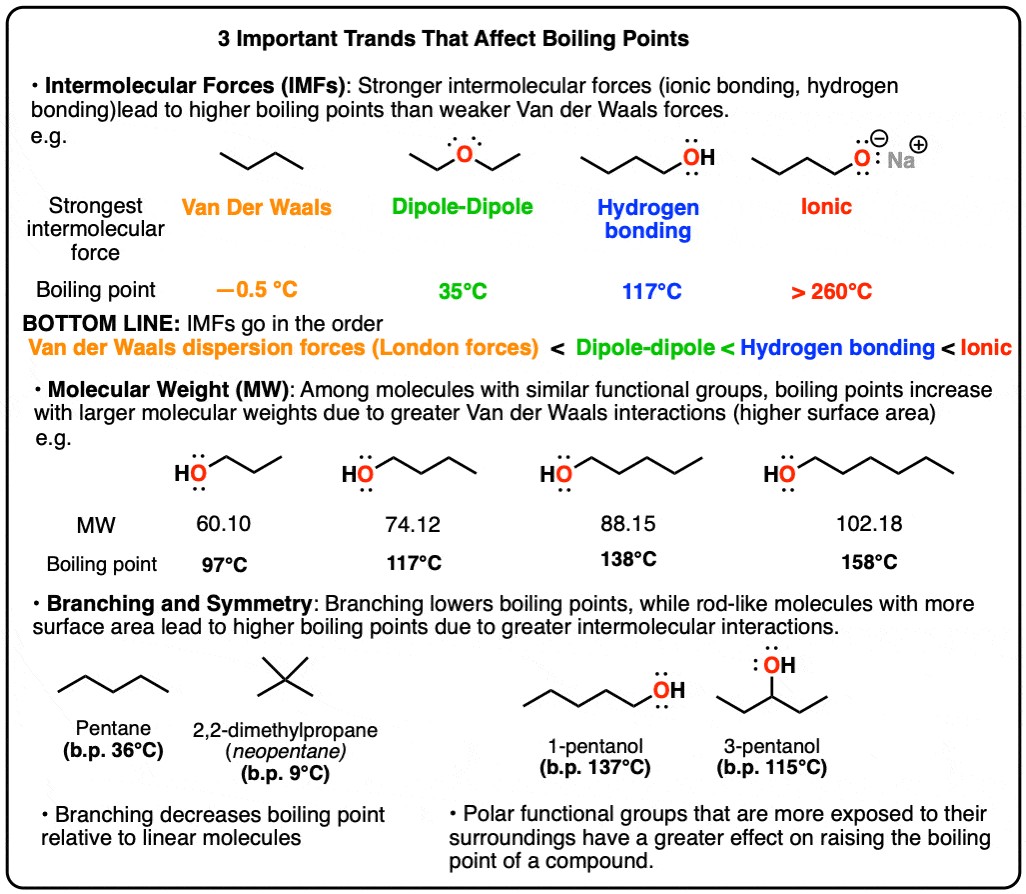
Intermolecular forces are the attractive or repulsive forces between neighboring molecules. These forces are fundamental in determining physical properties such as boiling point, melting point, viscosity, and surface tension. The stronger these forces, the more energy is required to overcome them, leading to higher boiling and melting points.
There are several types of intermolecular forces, each with varying strengths:
- Ionic Interactions: These are the strongest intermolecular forces, occurring between ions in ionic compounds. Ionic interactions result from the electrostatic attraction between oppositely charged ions.
- Hydrogen Bonding: This is a strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F).
- Dipole-Dipole Interactions: These forces occur between polar molecules that have a permanent dipole moment. The positive end of one molecule is attracted to the negative end of another.
- Van der Waals Dispersion Forces (London Dispersion Forces): These are the weakest intermolecular forces, present in all molecules, whether polar or nonpolar. They arise from temporary, instantaneous dipoles that occur due to the random movement of electrons.
1.2 Comparing Butane Alcohol Derivatives
Consider butane alcohol derivatives to illustrate the impact of intermolecular forces. Diethyl ether (C4H10O) molecules are held together by dipole-dipole interactions due to the polarized C-O bonds. 1-butanol, an isomer of diethyl ether, has a significantly higher boiling point (117°C) compared to diethyl ether (35°C). This difference is due to the presence of a hydroxyl group (-OH) in butanol, which allows for hydrogen bonding.
Sodium butoxide, a salt, exhibits even stronger attractive forces and decomposes before it can boil, highlighting the strength of ionic interactions. Butane (C4H10), lacking polar functional groups, relies solely on weak Van der Waals dispersion forces, resulting in a boiling point of 0°C, much lower than diethyl ether.
1.3 The Influence of Functional Groups
The functional groups present in a molecule play a crucial role in determining its boiling point, particularly among molecules with similar molecular weights. For example, consider amine and carboxylic acid isomers. Amines can form hydrogen bonds, but not as strongly as carboxylic acids, which can form stronger hydrogen bonds due to the presence of both a hydroxyl and a carbonyl group. This results in higher boiling points for carboxylic acids compared to amines of similar molecular weight.
2. The Impact of Molecular Weight on Boiling Point
For substances with a given functional group, boiling point generally increases with molecular weight. This trend is evident when comparing alkanes, alcohols, carboxylic acids, and ethers of increasing carbon chain length.
2.1 Van der Waals Dispersion Forces and Surface Area
Van der Waals dispersion forces are proportional to the surface area of a molecule. As molecular weight increases, so does the length of the carbon chain and, consequently, the surface area. This larger surface area allows for greater interaction between molecules, increasing the overall attractive forces and raising the boiling point. Long molecules can align closely, maximizing these interactions.
2.2 Comparing Alkanes, Alcohols, Carboxylic Acids, and Ethers
Consider the boiling points of various organic compounds. Alkanes, with only Van der Waals forces, have lower boiling points compared to alcohols, which exhibit hydrogen bonding. Carboxylic acids, capable of forming strong hydrogen bonds, have even higher boiling points. Ethers, with dipole-dipole interactions, fall in between alkanes and alcohols.
2.3 Molecular Weight and Intermolecular Forces
As molecular weight increases, the cumulative effect of intermolecular forces becomes more significant. For example, longer chain alkanes have higher boiling points because of the increased surface area available for Van der Waals interactions. These forces, although individually weak, collectively contribute significantly to the overall intermolecular attraction.
3. The Influence of Symmetry on Boiling and Melting Points
Molecular symmetry also plays a role in determining boiling and melting points. Molecules with a more symmetrical, rod-like shape can pack more closely together, leading to stronger intermolecular forces.
3.1 Symmetry and Surface Area
Rod-like molecules have a greater surface area available for intermolecular interactions compared to more spherical molecules. This increased surface area results in stronger Van der Waals dispersion forces, leading to higher boiling points.
3.2 Comparing Pentane and 2,2-Dimethylpropane
Consider the isomers pentane (boiling point 36°C) and 2,2-dimethylpropane (boiling point 9°C). Pentane, with its linear structure, has a larger surface area and can pack more closely with neighboring molecules, resulting in stronger Van der Waals forces and a higher boiling point compared to the more spherical 2,2-dimethylpropane.
3.3 Symmetry and Hydrogen Bonding in Alcohols
The position of functional groups, such as the hydroxyl group in alcohols, also affects boiling points. Alcohols with the hydroxyl group at the end of the carbon chain (e.g., 1-pentanol) tend to have higher boiling points than those with the hydroxyl group in the middle (e.g., 3-pentanol). This is because the terminal hydroxyl group is more exposed and can form stronger hydrogen bonds with neighboring molecules.
4. Detailed Examples and Comparisons
To further illustrate these principles, let’s examine several detailed examples and comparisons of different substances and their boiling points.
4.1 Comparing Isomers with Different Functional Groups
Isomers are molecules with the same molecular formula but different structural arrangements. The boiling points of isomers can vary significantly depending on the functional groups present and their positions within the molecule.
Example: Compare the boiling points of ethanol (C2H5OH) and dimethyl ether (CH3OCH3). Both compounds have the same molecular formula, but ethanol has a hydroxyl group capable of hydrogen bonding, while dimethyl ether only has dipole-dipole interactions. As a result, ethanol has a much higher boiling point (78.37°C) compared to dimethyl ether (-24°C).
4.2 Comparing Alkanes of Different Chain Lengths
Alkanes are hydrocarbons with only single bonds. The boiling points of alkanes increase with increasing carbon chain length due to the greater surface area available for Van der Waals dispersion forces.
Example: Compare the boiling points of methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10). Methane has a boiling point of -161.5°C, ethane -88.6°C, propane -42.1°C, and butane -0.5°C. The trend clearly shows that as the carbon chain length increases, the boiling point also increases.
4.3 Comparing Alcohols with Different Structures
Alcohols have the general formula ROH, where R is an alkyl group and OH is a hydroxyl group. The boiling points of alcohols are influenced by both the molecular weight and the structure of the alkyl group.
Example: Compare the boiling points of 1-butanol (CH3CH2CH2CH2OH), 2-butanol (CH3CH2CH(OH)CH3), and tert-butanol ((CH3)3COH). 1-butanol has a boiling point of 117.7°C, 2-butanol 99.5°C, and tert-butanol 82.4°C. The decrease in boiling point from 1-butanol to tert-butanol is due to the increasing branching, which reduces the surface area available for intermolecular interactions.
4.4 Comparing Carboxylic Acids
Carboxylic acids (RCOOH) exhibit strong hydrogen bonding due to the presence of both a hydroxyl and a carbonyl group. The boiling points of carboxylic acids are generally higher than those of alcohols and ketones with similar molecular weights.
Example: Compare the boiling points of acetic acid (CH3COOH), propionic acid (CH3CH2COOH), and butyric acid (CH3CH2CH2COOH). Acetic acid has a boiling point of 118°C, propionic acid 141°C, and butyric acid 163.5°C. The increase in boiling point with increasing carbon chain length is due to the greater Van der Waals dispersion forces.
5. Boiling Point Trends and Their Significance
Understanding the trends in boiling points is essential for various applications in chemistry, including predicting the physical properties of substances, designing chemical reactions, and separating compounds through distillation.
5.1 Predicting Physical Properties
By understanding the relationship between intermolecular forces, molecular weight, symmetry, and boiling points, chemists can predict the physical properties of unknown compounds. This is particularly useful in the development of new materials and pharmaceuticals.
5.2 Designing Chemical Reactions
Boiling points are important in designing chemical reactions. The boiling point of a solvent can influence the rate and selectivity of a reaction. For example, reactions that require high temperatures may need to be conducted in solvents with high boiling points.
5.3 Separating Compounds through Distillation
Distillation is a technique used to separate compounds based on their boiling points. By carefully controlling the temperature, compounds with different boiling points can be selectively vaporized and condensed, allowing for their separation. This technique is widely used in the petroleum industry to separate crude oil into its various components.
6. Advanced Concepts in Boiling Point Determination
While intermolecular forces, molecular weight, and symmetry are the primary factors influencing boiling points, there are other advanced concepts that can further refine our understanding.
6.1 Azeotropes
An azeotrope is a mixture of two or more liquids whose proportions cannot be altered by simple distillation. This occurs because, when an azeotrope is boiled, the vapor has the same proportions of constituents as the unboiled mixture. Azeotropes can be either minimum-boiling or maximum-boiling.
Example: A mixture of 95.6% ethanol and 4.4% water forms a minimum-boiling azeotrope with a boiling point of 78.1°C, which is lower than the boiling points of pure ethanol (78.4°C) and water (100°C).
6.2 Clathrate Hydrates
Clathrate hydrates are inclusion compounds in which small molecules, such as methane or ethane, are trapped within a crystal lattice of water molecules. The formation of clathrate hydrates can affect the boiling point and phase behavior of the mixture.
Example: Methane hydrate, in which methane molecules are trapped within a water lattice, is stable at low temperatures and high pressures. When methane hydrate is heated, the methane gas is released, which can affect the overall boiling point of the system.
6.3 Surface Tension and Boiling Point
Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. Liquids with strong intermolecular forces tend to have high surface tension, which can influence the boiling point.
Example: Water has a high surface tension due to its strong hydrogen bonding. This high surface tension contributes to its relatively high boiling point compared to other liquids with similar molecular weights.
7. Practical Applications of Boiling Point Knowledge
Understanding boiling points has numerous practical applications across various industries and scientific disciplines.
7.1 Chemical Industry
In the chemical industry, boiling points are essential for designing and optimizing chemical processes. They are used to select appropriate solvents, control reaction temperatures, and separate products through distillation.
Example: In the production of pharmaceuticals, distillation is used to purify drug compounds and remove unwanted byproducts. The boiling points of the desired product and the impurities are carefully considered to ensure efficient separation.
7.2 Petroleum Industry
The petroleum industry relies heavily on boiling points to separate crude oil into its various components, such as gasoline, kerosene, and diesel fuel. Fractional distillation, a process that separates compounds based on their boiling points, is a key technology in oil refining.
Example: Crude oil is heated in a distillation column, and the different components vaporize at different temperatures. These vapors are then condensed at different heights in the column, allowing for the separation of the various fractions.
7.3 Food and Beverage Industry
In the food and beverage industry, boiling points are important for processes such as cooking, brewing, and distillation of alcoholic beverages.
Example: In the distillation of whiskey, the boiling points of ethanol and water are carefully controlled to produce a spirit with the desired alcohol content. The distiller adjusts the temperature to selectively vaporize and condense the ethanol, which has a lower boiling point than water.
7.4 Environmental Science
Boiling points are also relevant in environmental science, particularly in the study of volatile organic compounds (VOCs). VOCs are organic compounds that easily vaporize at room temperature and can contribute to air pollution.
Example: Monitoring the boiling points of VOCs in the atmosphere can help scientists understand their sources, transport, and fate. This information is used to develop strategies for reducing air pollution and protecting public health.
8. Common Mistakes to Avoid When Predicting Boiling Points
When predicting boiling points, it is important to avoid common mistakes that can lead to inaccurate predictions.
8.1 Overlooking Intermolecular Forces
One common mistake is to overlook the importance of intermolecular forces. Even if two compounds have similar molecular weights, their boiling points can differ significantly if they have different types of intermolecular forces.
Example: Comparing propane (C3H8) and acetone (CH3COCH3). Acetone has a higher boiling point due to dipole-dipole forces.
8.2 Ignoring Molecular Shape
Molecular shape can also play a significant role in determining boiling points. Ignoring the shape of a molecule and its impact on surface area can lead to inaccurate predictions.
Example: Comparing n-pentane (linear) and neopentane (spherical). N-pentane has a higher boiling point due to the greater surface area.
8.3 Not Considering Hydrogen Bonding
Hydrogen bonding is a particularly strong type of intermolecular force and should always be considered when predicting boiling points. Failing to recognize the potential for hydrogen bonding can lead to significant errors.
Example: Comparing diethyl ether and butanol. Butanol has a higher boiling point due to hydrogen bonding.
9. Tools and Resources for Comparing Boiling Points
Several tools and resources are available to help students and professionals compare boiling points and understand the factors that influence them. COMPARE.EDU.VN provides comprehensive comparison tools to aid in this process.
9.1 Online Databases
Online databases such as the NIST Chemistry WebBook and ChemSpider provide boiling point data for a wide range of compounds. These databases can be used to quickly look up the boiling points of specific substances and compare them to each other.
9.2 Software Tools
Software tools such as ChemDraw and MarvinSketch can be used to draw chemical structures and predict their physical properties, including boiling points. These tools use algorithms based on the principles of intermolecular forces and molecular structure to estimate boiling points.
9.3 Textbooks and Reference Books
Textbooks and reference books on organic chemistry and physical chemistry provide detailed explanations of the factors that influence boiling points and offer examples of how to predict them. These resources can be valuable for students and professionals who want to deepen their understanding of this topic.
10. Frequently Asked Questions (FAQ)
Q1: What is the primary factor that determines the boiling point of a substance?
The primary factor is the strength of intermolecular forces between molecules. Stronger forces require more energy to overcome, resulting in higher boiling points.
Q2: How does molecular weight affect boiling point?
Generally, as molecular weight increases, boiling point also increases due to greater Van der Waals dispersion forces.
Q3: What role does molecular shape play in determining boiling point?
More symmetrical, rod-like molecules have higher boiling points because they can pack more closely together, leading to stronger intermolecular forces.
Q4: How do functional groups influence boiling point?
Functional groups influence the type and strength of intermolecular forces. For example, alcohols have higher boiling points than alkanes due to hydrogen bonding.
Q5: What are Van der Waals dispersion forces?
Van der Waals dispersion forces are weak, temporary attractions between molecules resulting from instantaneous dipoles caused by electron movement.
Q6: Why do carboxylic acids have higher boiling points than alcohols with similar molecular weights?
Carboxylic acids form stronger hydrogen bonds due to the presence of both a hydroxyl and a carbonyl group, leading to higher boiling points.
Q7: How can boiling points be used in the chemical industry?
Boiling points are used to select appropriate solvents, control reaction temperatures, and separate products through distillation.
Q8: What is an azeotrope?
An azeotrope is a mixture of liquids that boils at a constant temperature and has the same composition in the vapor phase as in the liquid phase.
Q9: How does hydrogen bonding affect boiling point?
Hydrogen bonding significantly increases boiling point because it is a strong type of intermolecular force.
Q10: Where can I find reliable boiling point data for different substances?
Reliable data can be found in online databases like the NIST Chemistry WebBook and ChemSpider, as well as in chemistry textbooks and reference books.
Understanding the boiling points of substances involves considering intermolecular forces, molecular weight, and molecular symmetry. By comparing these factors, one can predict and explain the boiling points of different compounds. Whether you are a student or a professional, COMPARE.EDU.VN can help you navigate these complex concepts and make informed decisions.
Ready to dive deeper and make smarter comparisons? Visit compare.edu.vn today to explore our comprehensive resources and tools. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090.