A Peculiarity When Compared To other mutations in the hydrophobic pocket of BRAF is the BRAF V600E mutation, a key area of focus for COMPARE.EDU.VN. This striking example in the oncogenic serine/threonine kinase BRAF helps one understand how a specific change affects the disease. Explore the nuances of BRAF mutations, kinase activation, and the broader implications for personalized medicine. Delve into single nucleotide substitutions, kinase activity, and the random forest classifier.
1. Understanding BRAF and Its Role
BRAF, a serine/threonine protein kinase, plays a crucial role in regulating cellular responses, including cell division and differentiation, primarily through the MEK/ERK signaling pathway. Its involvement spans from germline diseases like cardiofaciocutaneous and Noonan syndromes to somatic cancers affecting the thyroid, skin, colon, and lung. BRAF’s structure consists of an N-terminal region with a Ras-binding domain, followed by a cysteine-rich motif, and a C-terminal kinase domain. This intricate structure enables BRAF to regulate cell growth and differentiation.
1.1. BRAF’s Autoinhibition Mechanism
BRAF is naturally autoinhibited, adopting a closed conformation. This autoinhibition is achieved through the interaction of the N-terminal conserved region with the kinase domain, mediated by the phosphorylated residues Ser365 and Ser729, along with a 14-3-3 dimer. Dephosphorylation of Ser365 allows the Ras-binding domain to interact with Ras at the plasma membrane, which releases the autoinhibition. Subsequent phosphorylation at Thr599 and Ser602 in the activation segment (AS) activates the kinase.
1.2. Kinase Domain Subdomains
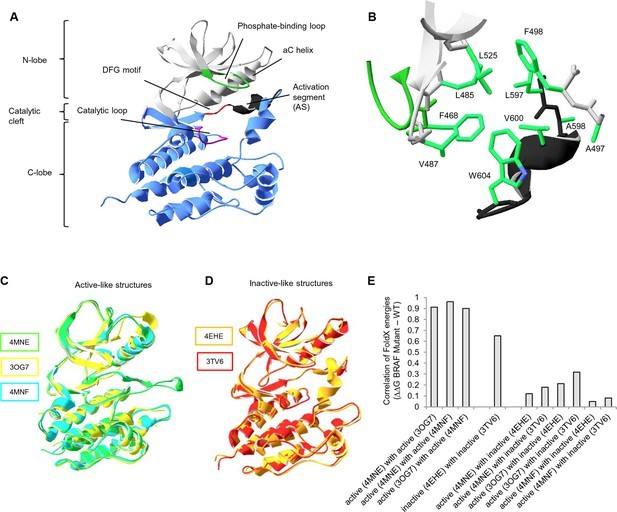
The BRAF kinase domain is composed of two subdomains: a small N-terminal lobe and a large C-terminal lobe. The N-terminal lobe contains the nucleotide-binding pocket and the phosphate-binding loop, while the C-terminal lobe binds protein substrates and contains the catalytic loop. These lobes move relative to each other, opening or closing the cleft. The AS residues interact with the phosphate-binding loop and the αC helix, forming a ‘hydrophobic pocket’ that locks the kinase in its inactive state.
2. The Significance of BRAF V600E Mutation
The V600E mutation in BRAF’s kinase AS is remarkably prevalent, accounting for over 95% of all BRAF cancer mutations. This hotspot mutation has significant implications for cancer development and treatment strategies. BRAF V600E has an increased propensity to form dimers, contributing to its constitutive kinase activity.
2.1. Prevalence and Location
Mutations in the phosphate-binding loop (residues 464 to 472) constitute less than 1% of all BRAF mutations in cancer. However, the AS, particularly at position Val600, is a major hotspot. The V600E mutation is by far the most common somatic cancer mutation in BRAF.
2.2. Impact on Hydrophobic Pocket
In the inactive conformation, Val600 is nestled within a hydrophobic pocket created by residues from the N-terminal subdomain and the AS. When Val600 is substituted by a charged amino acid like Glutamic acid (Glu), this hydrophobic interaction is disrupted, leading to constitutive kinase activation. This disruption bypasses the need for RAF dimerization or interaction with Ras for activation.
3. Why V600E is a Peculiarity Compared to Other Mutations
Extensive research has illuminated the role of BRAF kinase and the activity of V600E. However, it remains unclear why other amino acid substitutions in the hydrophobic pocket are not as frequently observed in cancer. For instance, mutating Leu597 to Glu or V487 to Glu could also potentially release the AS and cause kinase activation.
3.1. Structure-Energy Analysis
To investigate this, combined structure-energy, experimental, and statistical analyses were conducted on mutations within the hydrophobic pocket. The findings reveal that V600E is unique because it opens the AS by destabilizing autoinhibitory interactions without significantly impairing the folding of either the inactive or active kinase domain.
3.2. Single Nucleotide Substitution Advantage
V600E results from a single nucleotide substitution, whereas other mutations like V600D, V600K, and V600R require two nucleotide substitutions. Mutations needing three base substitutions, such as V600H, exhibit kinase activities comparable to V600E.
4. Comprehensive Analysis of Hydrophobic Pocket Mutations
A comprehensive approach is essential to understand why V600E stands out. This involves structure-energy calculations, experimental validations, and statistical analyses of various mutations within the hydrophobic pocket.
4.1. Examining Alternative Mutations
Consider mutations like Leu597 to Glu or Val487 to Glu. These alterations could theoretically disrupt the hydrophobic pocket and activate the kinase. However, these mutations are not commonly found in cancer. Understanding why these mutations are rare requires a detailed examination of their impact on protein structure and function.
4.2. Role of Single Nucleotide Substitutions
The fact that V600E arises from a single nucleotide substitution is significant. Mutations requiring multiple substitutions are less likely to occur spontaneously. This difference in mutational accessibility contributes to the prevalence of V600E.
5. Methodology: Structure-Energy, Experimental, and Statistical Analyses
The investigation into the uniqueness of V600E involved a combination of computational and experimental techniques. Structure-energy calculations provided insights into the stability of different BRAF conformations with various mutations. Experimental assays measured the kinase activity of these mutants. Statistical analyses helped quantify the importance of different parameters affecting mutation frequencies.
5.1. Structure-Energy Calculations
These calculations assess the impact of mutations on the stability of BRAF’s active and inactive states. By quantifying the energy changes associated with each mutation, it is possible to predict whether a given mutation will favor kinase activation or destabilize the protein.
5.2. Experimental Validations
In vitro kinase assays are used to measure the activity of BRAF mutants. These assays provide direct evidence of whether a mutation results in increased kinase activity. Cell-based assays can also be used to assess the effects of mutations on cell growth and signaling.
5.3. Statistical Analyses
Statistical methods, such as random forest classifiers, are used to evaluate the importance of various factors contributing to BRAF cancer mutation frequencies. These factors include the energy changes associated with mutations, their mutational accessibility, and their impact on protein folding.
6. Key Findings: The Uniqueness of V600E
The research demonstrates that V600E is the only single nucleotide substitution that effectively opens the AS by destabilizing autoinhibitory interactions without compromising the structural integrity of the kinase domain.
6.1. Destabilization of Autoinhibition
V600E disrupts the hydrophobic pocket, destabilizing the interaction between the AS and the N-terminal lobe. This destabilization releases autoinhibition, allowing the kinase to become constitutively active.
6.2. Preservation of Kinase Domain Folding
Unlike other mutations, V600E does not significantly impair the folding of the kinase domain. This is crucial because a properly folded kinase domain is necessary for enzymatic activity. Mutations that disrupt folding can render the kinase inactive, even if they also release autoinhibition.
7. Other Mutations Requiring Three Base Substitutions
Mutations needing three base substitutions, such as V600H, exhibit kinase activities similar to V600E. However, their lower probability of occurrence makes them less frequent in cancer.
7.1. Kinase Activity of V600H
The V600H mutation, which requires three nucleotide changes, displays comparable kinase activity to V600E. This indicates that the specific amino acid substitution at position 600 is critical for kinase activation.
7.2. Low Probability of Occurrence
The rarity of V600H and other mutations requiring multiple base changes is due to the low probability of these events occurring spontaneously. This mutational constraint explains why V600E is the predominant BRAF mutation in cancer.
8. Quantitative Measures for Cancer Mutation Frequencies
The study provides a quantitative measure for all parameters that influence BRAF cancer mutation frequencies. This measure is obtained by evaluating the importance of different factors using a random forest classifier.
8.1. Random Forest Classifier
A random forest classifier is a machine learning algorithm that can identify the most important predictors of a particular outcome. In this case, the classifier is trained to predict BRAF cancer mutation frequencies based on factors such as energy changes, mutational accessibility, and protein folding.
8.2. Evaluating the Importance of Parameters
By analyzing the random forest classifier, it is possible to determine which parameters have the greatest impact on BRAF cancer mutation frequencies. This information can be used to develop a more complete understanding of the factors driving cancer development.
9. Implications for Other Kinases and Disease-Causing Proteins
The findings from this study can be extended to other kinases and disease-causing proteins, especially if high-resolution X-ray structures are available. The approach of combining structure-energy calculations, experimental validations, and statistical analyses can be applied to understand the effects of mutations in other proteins.
9.1. Application to Other Kinases
Many kinases share structural similarities with BRAF. The principles learned from studying BRAF mutations can be applied to understand mutations in other kinases that are implicated in cancer and other diseases.
9.2. Generalizability to Disease-Causing Proteins
The combined approach used in this study is broadly applicable to understanding the effects of mutations in disease-causing proteins. By integrating computational and experimental data, it is possible to gain insights into the mechanisms by which mutations lead to disease.
10. Understanding the BRAF Hydrophobic Pocket
The hydrophobic pocket in BRAF is a critical region for regulating kinase activity. This pocket is formed by residues from both the N-terminal subdomain and the AS. Mutations within this pocket can disrupt the interactions that maintain BRAF in its inactive state, leading to constitutive activation.
10.1. Residues Forming the Hydrophobic Pocket
The hydrophobic pocket is composed of residues such as Ala497, Phe498, Leu525, Leu485, Phe468, and Val487 from the N-terminal subdomain, along with Leu597, Ala598, and Trp604 from the AS. These residues create a nonpolar environment that stabilizes the inactive conformation of BRAF.
10.2. Impact of Mutations on the Pocket
Mutations that introduce charged amino acids into the hydrophobic pocket can disrupt the nonpolar environment, leading to destabilization of the inactive state. This destabilization can result in constitutive kinase activation, as seen with the V600E mutation.
11. Role of RAF Dimerization and Ras Interaction
BRAF V600E does not require RAF dimerization or interaction with Ras to be active. However, it exhibits an increased propensity to form dimers.
11.1. Independence from RAF Dimerization
Unlike wild-type BRAF, which requires dimerization with other RAF family members for activation, V600E is active even in the absence of dimerization. This independence from dimerization contributes to its constitutive activity.
11.2. Independence from Ras Interaction
Similarly, V600E does not require interaction with Ras for activation. This is significant because Ras is a key regulator of BRAF activity. By bypassing the need for Ras interaction, V600E can activate the MEK/ERK pathway even when Ras signaling is not active.
12. Clinical Significance of BRAF Mutations
BRAF mutations, particularly V600E, are clinically significant because they are associated with increased cancer risk and can influence treatment strategies.
12.1. Association with Cancer Risk
The presence of BRAF mutations is associated with an increased risk of developing certain types of cancer. This is because these mutations can drive uncontrolled cell growth and proliferation.
12.2. Impact on Treatment Strategies
The identification of BRAF mutations can influence treatment strategies. For example, patients with BRAF-mutated cancers may benefit from targeted therapies that specifically inhibit BRAF kinase activity.
13. The Importance of High-Resolution X-Ray Structures
High-resolution X-ray structures are essential for understanding the effects of mutations on protein structure and function. These structures provide detailed information about the three-dimensional arrangement of atoms in a protein, which can be used to predict how mutations will affect protein stability and activity.
13.1. Providing Detailed Structural Information
X-ray structures provide a high-resolution view of protein structure, allowing researchers to visualize the positions of individual atoms. This level of detail is crucial for understanding how mutations alter protein structure and function.
13.2. Predicting Mutation Effects
By analyzing X-ray structures, it is possible to predict how mutations will affect protein stability and activity. This information can be used to guide experimental studies and to develop targeted therapies.
14. Statistical Analysis with Random Forest Classifier
Statistical analysis using a random forest classifier helps in evaluating the importance of various parameters that contribute to BRAF cancer mutation frequencies.
14.1. Identifying Key Predictors
The random forest classifier identifies key predictors of BRAF cancer mutation frequencies. These predictors include energy changes associated with mutations, mutational accessibility, and the impact on protein folding.
14.2. Quantifying Parameter Importance
By quantifying the importance of each parameter, the random forest classifier provides insights into the factors driving cancer development. This information can be used to develop more effective prevention and treatment strategies.
15. Conclusion: Why V600E Remains a Key Focus
In summary, the BRAF V600E mutation’s peculiarity when compared to other mutations stems from its unique combination of factors: it opens the AS by destabilizing autoinhibitory interactions, it doesn’t significantly impair the folding of the kinase domain, and it arises from a single nucleotide substitution. These findings are crucial for understanding cancer biology and for developing targeted therapies.
This comprehensive analysis highlights the importance of considering structural, energetic, and statistical factors when studying the effects of mutations. The insights gained from this research can be applied to other kinases and disease-causing proteins, providing a framework for understanding the molecular basis of disease.
Looking for more detailed comparisons to make informed decisions? Visit COMPARE.EDU.VN at 333 Comparison Plaza, Choice City, CA 90210, United States, or reach out via Whatsapp at +1 (626) 555-9090 for expert insights and detailed analyses. Let us help you make the right choice!
Frequently Asked Questions (FAQ)
1. What is BRAF?
BRAF is a serine/threonine protein kinase involved in the MEK/ERK signaling pathway, regulating cell division and differentiation.
2. What is the BRAF V600E mutation?
It is a specific mutation in the BRAF kinase activation segment (AS) where valine (V) at position 600 is replaced by glutamic acid (E).
3. Why is V600E the most common BRAF mutation in cancer?
It results from a single nucleotide substitution, making it more likely to occur, and it destabilizes autoinhibition without impairing kinase domain folding.
4. How does the V600E mutation affect BRAF activity?
It disrupts the hydrophobic pocket, leading to constitutive kinase activation, bypassing the need for RAF dimerization or Ras interaction.
5. What is the hydrophobic pocket in BRAF?
It is a region formed by residues from the N-terminal subdomain and the AS, stabilizing the inactive conformation of BRAF.
6. What are the clinical implications of BRAF mutations?
They are associated with increased cancer risk and influence treatment strategies, often targeted with specific BRAF inhibitors.
7. How do structure-energy calculations help in studying BRAF mutations?
They assess the impact of mutations on the stability of BRAF’s active and inactive states, predicting kinase activation or destabilization.
8. What is a random forest classifier used for in this context?
It evaluates the importance of various parameters, like energy changes and mutational accessibility, in contributing to BRAF cancer mutation frequencies.
9. Can the findings of this study be applied to other kinases?
Yes, the principles and methods can be extended to other kinases and disease-causing proteins, especially with high-resolution X-ray structures.
10. Where can I find more detailed comparisons of medical treatments and options?
Visit compare.edu.vn for comprehensive analyses and expert insights to help you make informed decisions.