A Comparative Design Using Concurrent Controls evaluates different interventions simultaneously, offering insights into their relative effectiveness. On COMPARE.EDU.VN, you can explore detailed comparisons and analysis of various products, services, and strategies. This ensures you make informed decisions and achieve optimal outcomes. Enhance your understanding of comparative designs by learning about randomized controlled trials, factorial designs, and more, equipping you with the knowledge to navigate complex choices confidently.
1. Understanding Comparative Design with Concurrent Controls
1.1. What is a Comparative Design Using Concurrent Controls?
A comparative design using concurrent controls is a research methodology that evaluates the effectiveness of one or more interventions by comparing them to a control group simultaneously within the same time frame. This approach allows researchers to assess the relative efficacy of different treatments or strategies under similar conditions. Concurrent controls, which are typically either a placebo or a standard treatment, are administered alongside the experimental intervention to provide a benchmark for comparison. This design is commonly used in clinical trials, product testing, and policy evaluations.
1.2. Why Use Concurrent Controls?
Using concurrent controls offers several advantages in research:
- Reduced Bias: By evaluating interventions and controls concurrently, the risk of bias from external factors (e.g., changes in the environment or population) is minimized.
- Accurate Comparisons: Concurrent controls provide a reliable baseline, allowing for more accurate comparisons between the interventions being studied.
- Efficiency: This design can be more efficient than historical control designs, which compare current interventions to past data, as it eliminates the need to account for temporal changes.
- Validity: Concurrent controls enhance the internal validity of the study, ensuring that the observed effects are due to the intervention and not extraneous variables.
1.3. Key Components of a Comparative Design
A comparative design using concurrent controls typically includes the following components:
- Experimental Group: The group receiving the new or experimental intervention.
- Control Group: The group receiving a placebo or standard treatment (concurrent control).
- Randomization: Participants are randomly assigned to either the experimental or control group to ensure that the groups are comparable at baseline.
- Blinding: Whenever possible, participants and researchers are blinded to the treatment assignment to reduce bias.
- Concurrent Administration: Interventions and controls are administered simultaneously during the study period.
- Outcome Measurement: Standardized measures are used to assess the outcomes of interest in both groups.
2. Types of Comparative Designs
2.1. Parallel Group Design
The parallel group design is one of the most commonly used study designs. In this design, subjects are randomized to one or more study arms, with each arm receiving a different intervention. After randomization, each participant remains in their assigned treatment arm for the duration of the study.
Alt Text: Diagram illustrating the flow of participants in a parallel arm design, showing randomization and allocation to either test drug or control arm.
2.1.1. Steps Involved in a Parallel Arm Trial Design
- Eligibility Assessment: Determining whether potential subjects meet the criteria for inclusion in the study.
- Recruitment and Consent: Enrolling eligible subjects into the study after obtaining informed consent.
- Randomization: Randomly assigning subjects to either the test drug arm or the control arm.
- Allocation: Assigning the appropriate intervention (test drug or control) to each subject based on their group assignment.
2.1.2. Illustrative Example
Consider a comparative trial of Acitretin and Apremilast in palmoplantar psoriasis, where clinical equipoise exists regarding efficacy. This can be conducted as a randomized controlled parallel arm trial design, using Acitretin as an active control.
2.2. Cross-Over Design
In a cross-over design, participants receive both interventions (drug A and drug B) but in a different order. Some participants start with drug A and then switch to drug B (AB sequence), while others start with drug B and then switch to drug A (BA sequence). An adequate washout period is necessary before the crossover to eliminate the effects of the initially administered intervention.
Alt Text: Illustration of a cross-over trial design, demonstrating how participants switch between drug A and drug B with a washout period in between.
2.2.1. Advantages of Cross-Over Design
- Requires a smaller sample size.
- Each patient serves as their own control, balancing covariates in treatment and control arms.
2.2.2. Requirements for Cross-Over Design
- The disease must be chronic, stable, and incurable, with characteristics that do not vary during the study.
- The effect of each drug must be reversible.
2.2.3. Considerations for Cross-Over Design
| Consideration | Description |
|---|---|
| Carry-over Effects | The effects of the intervention during the first period should not carry over into the second period. If carry-over effects are suspected, more complex sequences are needed, which increase study duration and the chance of dropouts. |
| Treatment Effect | The treatment effect should be relatively rapid in onset with rapid reversibility of effect. |
| Disease Stability | The disease must be chronic, stable, and non-self-resolving. This design is typically avoided in vaccine trials because the immune system is permanently affected. The patient’s health status must be identical at the beginning of each treatment period. |
| Period Effect | Changes in patient characteristics due to the effect of the first drug or extraneous factors can lead to the ‘period effect.’ The internal and external trial environment must remain constant over time to reduce this effect. |
| Washout Period | Before the crossover, a drug-free washout period is needed for complete reversibility of the drug effect administered in the first period to avoid cumulative or subtractive effects. An accepted convention for the washout period is five half-lives of the drug involved. |
| Treatment Period Duration | Each treatment period must provide adequate time for the intervention to act meaningfully. |
| Sensitivity to Dropouts | The trial power is sensitive to dropouts due to the longer anticipated duration of the trial. |
| Identification of Adverse Events | Identifying the culprit drug for delayed adverse events during the later period of the study becomes difficult. |

2.2.4. Variations of Cross-Over Designs
- Switch Back Design (ABA vs BAB arms): Drug A → Drug B → Drug A in one arm versus Drug B → Drug A → Drug B in the other arm.
- N of 1 Design: Also known as “single-subject” or “structured within-patient randomized controlled multi-crossover trial design,” used to evaluate interventions in a single patient.
2.3. Factorial Design (2 × 2 Design)
A factorial design is suited for studying two or more interventions in various combinations within one study setting. It helps in studying interactive effects resulting from combinations of interventions.
Alt Text: Diagram illustrating a 2×2 factorial design, showing the combinations of treatment A, treatment B, and placebo.
2.3.1. How Factorial Design Works
In a 2 × 2 factorial design with a placebo, patients are randomized into four groups:
- Treatment A plus placebo
- Treatment B plus placebo
- Both treatments A and B
- Placebo only
Outcomes are analyzed using a two-way analysis of variance (ANOVA), comparing patients who receive treatment A with those who do not, and patients who receive treatment B with those who do not.
2.3.2. Advantages of Factorial Design
- Can answer two or more research questions with one trial.
- Reduces the sample size requirement by almost 50% compared to conducting separate trials for drug A and drug B.
2.3.3. Limitations of Factorial Design
- Requires that there is no interaction between treatments A and B.
- Complex protocols and statistical analytical complexities.
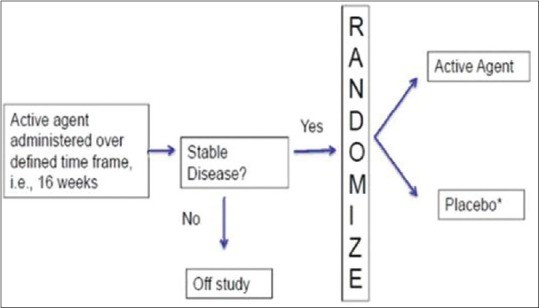
2.4. Randomized Withdrawal Design [Enrichment Enrolment Randomized Withdrawal (EERW)]
In this design, after an initial open-label period (enrichment period) where all subjects receive the intervention, non-responders are dropped from the trial. The responders (the enriched population) are then randomized to receive either the intervention or a placebo in the second phase of the trial.
Alt Text: Flowchart of an enrichment enrollment randomized withdrawal design, showing the enrichment phase and subsequent randomization of responders.
2.4.1. Advantages of Randomized Withdrawal Design
- Reduces time on placebo since only responders are randomized to placebo.
- Allows assessment of whether treatment needs to be continued or can be stopped.
2.4.2. Illustrative Example
Subjects with psoriasis vulgaris are initiated on a biologic, and those who achieve PASI 75 response at 16 weeks are continued. Non-PASI 75 achievers are dropped. PASI 75 responders are continued on the drug or assigned to a placebo, and retention of PASI 75 response at 1 year is compared between the two arms.
2.4.3. Disadvantages of Randomized Withdrawal Design
- Missing data due to withdrawals.
- Carry-over effects from the enrichment phase.
- Restriction to responders can affect the external validity of results.
- The treatment effect is overestimated since only responders are included.
- Not suitable for unpredictable diseases or those with slow evolution.
3. Applications of Comparative Designs
3.1. Clinical Trials
Comparative designs are extensively used in clinical trials to evaluate the efficacy and safety of new drugs, therapies, and medical devices. By comparing the outcomes of patients receiving the experimental treatment with those receiving a placebo or standard care, researchers can determine the clinical benefit of the new intervention.
3.2. Product Testing
In the field of product development, comparative designs are used to assess the performance and usability of different products. For example, manufacturers may conduct comparative studies to evaluate the durability, efficiency, and user satisfaction of different models of appliances or electronic devices.
3.3. Policy Evaluation
Comparative designs are also valuable in evaluating the effectiveness of public policies and social programs. By comparing outcomes in areas where a policy has been implemented with those in control areas, policymakers can assess the impact of the policy and make informed decisions about its continuation or modification.
3.4. Educational Interventions
In education, comparative designs can be used to compare the effectiveness of different teaching methods or curricula. For example, researchers may conduct studies to evaluate whether a new instructional approach leads to improved student learning outcomes compared to traditional methods.
4. Advantages of Comparative Designs
4.1. Objectivity
Comparative designs provide an objective framework for evaluating the relative merits of different options. By using standardized measures and statistical analysis, researchers can minimize bias and ensure that the findings are based on empirical evidence.
4.2. Identification of Best Practices
Comparative designs can help identify best practices by highlighting the interventions or strategies that produce the most favorable outcomes. This information can be used to inform decision-making and promote the adoption of effective practices in various fields.
4.3. Cost-Effectiveness
By comparing the costs and benefits of different options, comparative designs can help decision-makers identify the most cost-effective solutions. This is particularly important in areas such as healthcare and public policy, where resources are often limited.
4.4. Innovation
Comparative designs can stimulate innovation by encouraging the development and testing of new interventions and strategies. The process of comparing different options can lead to new insights and ideas that ultimately improve outcomes.
5. Disadvantages and Limitations
5.1. Complexity
Designing and implementing comparative studies can be complex, requiring expertise in research methodology, statistics, and the specific area being studied. This complexity can make it challenging for non-experts to conduct rigorous comparative evaluations.
5.2. Ethical Considerations
In some cases, ethical considerations may limit the use of comparative designs. For example, it may be unethical to withhold treatment from a control group if there is strong evidence that the intervention being studied is effective.
5.3. Resource Intensive
Comparative studies can be resource-intensive, requiring significant investments in time, personnel, and equipment. This can make it difficult to conduct large-scale comparative evaluations, particularly in resource-constrained settings.
5.4. Generalizability
The findings of comparative studies may not always be generalizable to other settings or populations. Factors such as differences in context, participant characteristics, and implementation strategies can affect the outcomes of interventions.
6. Implementing Comparative Design with Concurrent Controls
6.1. Define Objectives and Scope
Clearly define the objectives of the comparative design. What specific interventions are being compared? What outcomes are being measured? What is the scope of the study (e.g., target population, geographic area)?
6.2. Select Appropriate Design
Choose the most appropriate comparative design based on the research question and available resources. Consider parallel group, cross-over, factorial, or randomized withdrawal designs.
6.3. Randomization and Blinding
Implement randomization to assign participants to experimental and control groups. Use blinding techniques (single, double, or triple blinding) to minimize bias.
6.4. Data Collection and Analysis
Use standardized data collection methods to ensure consistency and accuracy. Employ appropriate statistical analysis techniques to compare outcomes between groups.
6.5. Ethical Considerations
Address all ethical considerations, including informed consent, protection of privacy, and minimization of risks to participants.
7. Real-World Examples
7.1. Comparing Different Cancer Treatments
In oncology, comparative designs are used to compare the effectiveness of different chemotherapy regimens, radiation therapy techniques, and targeted therapies. These studies help oncologists identify the most effective treatment options for different types of cancer.
7.2. Evaluating Different Diabetes Management Strategies
Comparative designs are used to evaluate the effectiveness of different diabetes management strategies, such as lifestyle interventions, medications, and insulin delivery systems. These studies help healthcare providers develop personalized treatment plans for patients with diabetes.
7.3. Assessing Different Approaches to Weight Loss
Comparative designs are used to compare the effectiveness of different weight loss programs, diets, and exercise regimens. These studies help individuals and healthcare providers identify the most effective strategies for achieving and maintaining a healthy weight.
7.4. Evaluating Different Investment Strategies
In finance, comparative designs can be used to evaluate the performance of different investment strategies, such as stocks, bonds, and real estate. These studies can help investors make informed decisions about how to allocate their capital.
8. Future Trends in Comparative Designs
8.1. Adaptive Designs
Adaptive designs allow for modifications to the trial based on interim data. This can increase efficiency and flexibility.
8.2. Personalized Medicine
Comparative designs can be tailored to individual patient characteristics, leading to more personalized treatment strategies.
8.3. Real-World Evidence (RWE)
Using real-world data from electronic health records and other sources to complement traditional clinical trials.
9. The Role of COMPARE.EDU.VN
9.1. Providing Comprehensive Comparisons
COMPARE.EDU.VN offers detailed and objective comparisons across a wide range of products, services, and ideas. Our platform provides the information you need to make informed decisions, saving you time and effort.
9.2. Empowering Informed Decisions
We empower users to make confident decisions by providing clear, concise, and evidence-based comparisons. Whether you’re comparing consumer products, educational programs, or healthcare options, COMPARE.EDU.VN is your go-to resource.
9.3. Supporting Diverse Needs
Our content is designed to meet the diverse needs of our users, from students and consumers to professionals and decision-makers. We provide information that is relevant, accessible, and tailored to your specific interests.
10. Frequently Asked Questions (FAQ)
10.1. What is the primary goal of a comparative design using concurrent controls?
The primary goal is to evaluate the effectiveness of different interventions by comparing them to a control group simultaneously, ensuring accurate and unbiased results.
10.2. How does randomization help in comparative designs?
Randomization ensures that participants are assigned to experimental and control groups randomly, making the groups comparable at baseline and reducing selection bias.
10.3. What is the importance of blinding in comparative studies?
Blinding minimizes bias by preventing participants and researchers from knowing the treatment assignment, which could influence the outcomes.
10.4. What are the key advantages of using concurrent controls?
Concurrent controls reduce bias, provide accurate comparisons, are efficient, and enhance the internal validity of the study.
10.5. What are some limitations of factorial designs?
Factorial designs require no interaction between treatments, and they involve complex protocols and statistical analysis.
10.6. In what situations is a cross-over design most appropriate?
A cross-over design is suitable for chronic, stable diseases where the treatment effect is reversible and a smaller sample size is desired.
10.7. What are the main challenges of randomized withdrawal designs?
The main challenges include missing data, carry-over effects, and limited external validity due to the restriction to responders.
10.8. How can COMPARE.EDU.VN help with decision-making?
COMPARE.EDU.VN offers comprehensive and objective comparisons across various domains, empowering users to make informed and confident decisions.
10.9. What ethical considerations should be addressed in comparative designs?
Ethical considerations include obtaining informed consent, protecting privacy, and minimizing risks to participants.
10.10. What are the future trends in comparative designs?
Future trends include adaptive designs, personalized medicine, and the use of real-world evidence to enhance the efficiency and relevance of comparative studies.
Comparative designs using concurrent controls are essential for evaluating the effectiveness of different interventions objectively. These designs, including parallel group, cross-over, factorial, and randomized withdrawal, offer unique advantages and are applied across various fields from clinical trials to policy evaluation. While there are challenges such as complexity and ethical considerations, the benefits of objectivity and identification of best practices make these designs invaluable. Explore COMPARE.EDU.VN for detailed comparisons and make well-informed decisions.
Ready to make smarter choices? Visit COMPARE.EDU.VN today to explore comprehensive comparisons and find the perfect solution for your needs. Our detailed analyses and objective evaluations empower you to make confident decisions. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or via WhatsApp at +1 (626) 555-9090. Start your journey to informed decision-making with compare.edu.vn.