A Color Atlas Of Comparative Pathology Of Pulmonary Tuberculosis is essential for understanding the disease across species. COMPARE.EDU.VN offers in-depth comparisons, highlighting key histopathological features. By examining these features across different animal models, a comprehensive resource can aid in better understanding and combating TB.
This article will explore the histopathological features of pulmonary tuberculosis in various animal models, discuss the key differences and similarities with human disease, and introduce the concept of a color atlas for comparative pathology. Discover practical information, expert analyses, and insightful comparisons – all in one place with COMPARE.EDU.VN.
1. What Is A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis?
A color atlas of comparative pathology of pulmonary tuberculosis is a detailed visual resource illustrating the histopathological features of pulmonary tuberculosis across different species. It serves as a reference guide, facilitating the comparison of disease manifestations in various animal models and humans.
Such an atlas aims to standardize the characterization and scoring of pulmonary lesions, aiding researchers and pathologists in accurately diagnosing and understanding tuberculosis. According to a study by the World Health Organization (WHO) in 2023, tuberculosis remains a leading cause of death from infectious diseases globally, highlighting the importance of resources like this atlas to advance our understanding and combat the disease. The atlas typically includes high-resolution images and detailed descriptions of granulomas, necrosis, and other pathological features.
1.1 Why Is A Color Atlas Necessary For Comparative Pathology?
A color atlas is indispensable for comparative pathology due to several reasons:
- Visual Standardization: It provides a standardized visual reference for the histopathological features of pulmonary tuberculosis across various species. This helps in reducing subjective interpretations and enhances diagnostic accuracy.
- Enhanced Diagnostic Accuracy: High-resolution images and detailed descriptions of granulomas, necrosis, and other pathological features enable more accurate identification and differentiation of lesions.
- Comparative Analysis: The atlas facilitates easy comparison of disease manifestations in different animal models and humans, aiding in the selection of appropriate models for specific research questions.
- Research Advancement: By standardizing the characterization of pulmonary lesions, the atlas promotes more consistent and reproducible research findings, accelerating progress in understanding and combating tuberculosis.
- Educational Resource: It serves as an invaluable educational tool for pathologists, researchers, and students, enhancing their knowledge of tuberculosis pathology and improving diagnostic skills.
1.2 How Does a Color Atlas Improve Tuberculosis Research?
A color atlas significantly enhances tuberculosis research through:
- Model Selection: It helps researchers select the most appropriate animal models by providing detailed comparative data on pathological similarities and differences with human disease.
- Drug Testing: Standardized visual references improve the accuracy of assessing drug efficacy in preclinical studies by facilitating precise evaluation of lesion regression and pathological changes.
- Vaccine Development: The atlas aids in evaluating vaccine candidates by enabling detailed comparison of immune responses and lesion characteristics in vaccinated versus unvaccinated animals.
- Pathogenesis Understanding: Visual comparisons of granuloma formation and disease progression in different models offer insights into the complex mechanisms of tuberculosis pathogenesis.
- Reproducibility: Standardized criteria for lesion characterization enhance the reproducibility of research findings, ensuring consistent results across different studies and laboratories.
1.3 Who Benefits From Using A Comparative Pathology Atlas?
The primary beneficiaries of a comparative pathology atlas include:
- Pathologists: Enhances diagnostic accuracy and provides a standardized reference for lesion characterization.
- Researchers: Aids in selecting appropriate animal models, evaluating drug efficacy, and understanding disease pathogenesis.
- Students: Serves as an educational resource for learning about tuberculosis pathology and comparative disease manifestations.
- Veterinarians: Helps in diagnosing and managing tuberculosis in animals, contributing to animal health and public health.
- Public Health Officials: Informs strategies for tuberculosis control and prevention by improving understanding of disease transmission and progression.
2. Tuberculosis in Humans: A Histopathological Overview
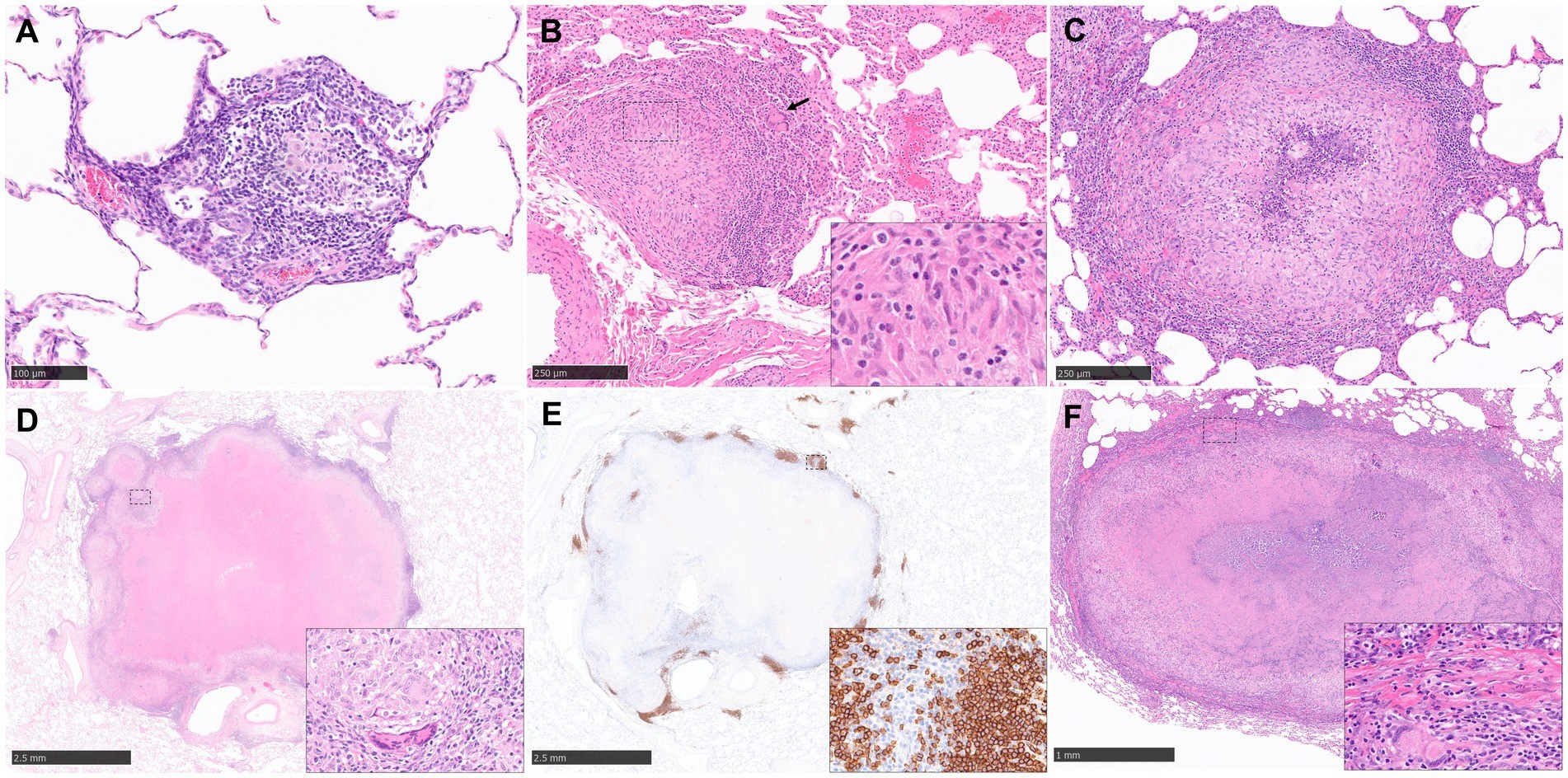
Early pulmonary granulomas in human lungs, induced by Mycobacterium tuberculosis (Mtb), feature a central region of large epithelioid cells (CD68+ by immunohistochemistry), surrounded by macrophages and predominantly CD4+ T cells, with fewer CD8+ T cells and multinucleated giant cells (MNGCs). Advanced human tuberculous granulomas are well-structured lesions characterized by a central necrotic area containing mycobacteria. This necrosis, resulting from the death of infected macrophages, is known as caseum due to its cheese-like consistency.
2.1 Key Features of Human Pulmonary Granulomas
The distinctive characteristics of human pulmonary granulomas encompass:
- Central Necrosis (Caseum): A core area of dead, infected macrophages, providing nutritional support for latent mycobacteria.
- Immune Cell Infiltration: A surrounding layer of T lymphocytes (mainly CD4+), macrophages, and MNGCs.
- Outer Layer of CD8+ T Cells: Large numbers of CD8+ T cells in the outer layers.
- B Cell Aggregates: Prominent B cell aggregates located more distally from the necrotic core, amidst T lymphocytes and mycobacteria-containing macrophages.
- Calcification and Fibrosis: As the disease progresses, the necrotic core can calcify and be encapsulated by a fibrotic rim.
2.2 How Ziehl-Neelsen Staining Detects Tuberculosis
Ziehl-Neelsen (ZN) staining is a routine technique used to identify acid-fast bacilli (AFB) in tissues. The process involves:
- Application of Carbolfuchsin: Tissue samples are stained with carbolfuchsin, a dye that binds to the mycolic acids in the cell walls of mycobacteria.
- Heat Treatment: The slide is heated to enhance dye penetration.
- Acid-Alcohol Decolorization: The sample is treated with acid-alcohol, which removes the dye from non-acid-fast bacteria and tissue.
- Counterstaining: Methylene blue is used as a counterstain, coloring non-acid-fast structures blue.
- Microscopic Examination: Acid-fast bacilli appear bright red against a blue background, allowing for easy identification.
2.3 Why Is It Difficult to Study Tuberculosis in Humans?
Studying tuberculosis in humans is challenging due to several factors:
- Limited Access to Tissues: Obtaining serial lung biopsies throughout disease progression is difficult.
- Altered Pathogenic Characteristics: Therapy and treatment inherently alter pathogenic characteristics and disease severity.
- Lack of Information on Granuloma Development: Acquiring lung samples from uncontrolled TB infections is difficult, limiting information on specific stages of granuloma development.
- Ethical Constraints: Invasive research procedures on human subjects pose ethical concerns.
3. Comparative Pathology of Animal Models
Animal models play a crucial role in tuberculosis research by providing insights into disease progression that are impossible to obtain from human studies. Various animal models, including non-human primates, rodents, guinea pigs, and rabbits, each offer unique advantages and limitations in replicating human TB disease.
3.1 Non-Human Primates as Tuberculosis Models
Old World monkeys (rhesus and cynomolgus macaques) and New World monkeys (common marmosets) are used as experimental models of human TB. These models develop the full range of Mtb disease observed in humans, from solid lesions to caseation, calcification, and cavitation.
3.1.1 How Disease Progression Varies in Macaques
Disease progression varies between macaque subspecies:
- Rhesus Macaques: More susceptible to Mtb infection.
- Cynomolgus Macaques: Can show active and latent TB infection.
- Common Marmosets: Different rates of disease progression and cavitation depending on the Mtb strain used.
- High-Dose Mtb Administration: Severe disease (acute TB).
- Low-Dose Mtb Administration: Asymptomatic infection, similar to latent TB in humans.
3.1.2 Describing Granuloma Stages in Active Tuberculosis
Active TB in NHP models exhibits a range of pulmonary granulomas:
- Early Lesions: Small aggregates of immune cells (macrophages, lymphocytes, neutrophils) lacking defined boundaries.
- Classical, Organized Granulomas: Abundant epithelioid macrophages, neutrophils, and some MNGCs without necrosis.
- Granulomas with Central Necrosis: Surrounded by a rim of epithelioid macrophages and a peripheral rim of lymphocytes.
- Large, Well-Defined Caseous Granulomas: With a central core of caseous necrosis.
- Cavitary Lesions: Rarely observed in Old World NHP models during active disease but noted in common marmosets infected with the CDC1551 strain.
3.1.3 How Scoring Systems Assess Disease Severity
Scoring systems are used to assess disease severity in NHP models:
- Lin et al. System: Based on granuloma type (caseous, solid, suppurative, mixed), cellular composition (presence/absence of lymphocytic cuff, mineralization, fibrosis, MNGCs, and epithelioid macrophages), and distribution pattern (focal, multifocal, coalescing, and invasive).
- Rayner et al. System: Uses different stages of development to categorize granulomas (stages 1–6), from small diffuse foci of cells to classical, well-demarcated granulomas with central caseous necrosis.
3.2 Rodents as Tuberculosis Models
Mice (Mus musculus) are widely used in TB immunology research due to abundant immunological tools and inbred strains. However, mice tend to develop an acute rather than chronic infection, and their lung granulomas lack the structured appearance of human granulomas.
3.2.1 Limitations of Granuloma Formation in Mice
Key limitations of granuloma formation in mice include:
- Lack of Caseous Necrosis: Most conventional mouse strains do not develop necrotic caseous centers.
- Foamy Macrophages: Infected macrophages show enlarged cytoplasm with high lipidic content (foamy macrophages).
- Absence of MNGCs: Tuberculous granulomas in mice lack MNGCs.
3.2.2 The Five Histopathological Stages of Granuloma Development
Rhoades et al. described five histopathological stages of granuloma development in mice:
- Category 1: Small, isolated lesions diffusely distributed, composed of thickened septae with mononuclear phagocytes and alveolar macrophages.
- Category 2: Scattered, discrete foci of alveolitis filled with mononuclear phagocytes, epithelioid, and foamy macrophages, with perivascular and peribronchiolar lymphocytes.
- Category 3: Moderate lesions with sheets of epithelioid and foamy macrophages filling the alveoli, mild interstitial fibrosis, and tight associations of lymphocytes; no central necrosis detected.
- Category 4: Enlarged, coalescing granulomatous lesions with macrophages, epithelioid macrophages, and large foamy macrophages; small foci of necrosis and polymorphonuclear cells with cholesterol clefts and advanced fibrosis.
- Category 5: Extensive chronic, interstitial fibrosing granulomas with thickened alveolar septae and demarcated areas filled with dead/dying epithelioid and foamy macrophages.
3.2.3 How “Humanized” Mice Model Human Tuberculosis
“Humanized” HIS-NSG mice, generated by transplanting human fetal liver-derived hematopoietic stem cells, can model human-like pulmonary granuloma initiation and formation, comprising solid non-necrotic granulomas, tuberculoid pneumonia, and caseous necrotic granulomas.
3.3 Guinea Pigs as Tuberculosis Models
Guinea pigs are extensively used in TB research due to many shared features with human disease. They develop large pulmonary granulomas with central caseating necrosis, surrounded by epithelioid macrophages, occasional MNGCs, and a rim of lymphocytes, along with chronic, fibrous encapsulation.
3.3.1 Early Lesion Development and Neutrophil Involvement
Small lesions comprising macrophages, neutrophils, and lymphocytes appear shortly after aerosol challenge, often located near large airways. Neutrophils facilitate early lesion growth through degranulation and release of hydrolytic enzymes.
3.3.2 Secondary Lesions and Their Distinctive Features
Secondary lesions in guinea pigs may arise from passive transportation of bacilli through pulmonary lymphatics or hematogenous spread. These lesions are generally small, predominantly lymphocytic, lack discrete granuloma morphology, and have reduced intra-lesional bacilli.
3.3.3 Staging Granuloma Development in Guinea Pigs
Our group has recently developed a new scoring system to differentiate morphological characteristics in a temporal pattern and which have been observed in both parenchyma and broncho-vascular, connective tissue:
- Stage I: Small, poorly demarcated collections of inflammatory cells (macrophages, lymphocytes, granulocytes).
- Stage II: Larger, circumscribed, well-demarcated, non-necrotic lesions (epithelioid macrophages, scattered lymphocytes, variable neutrophils).
- Stage III: Central necrosis visible, often with caseation.
- Stage IV: Granulomas with variable, often extensive, central dystrophic calcification and caseating necrosis.
3.4 Rabbits as Tuberculosis Models
The rabbit model is considered valuable for studying immunopathogenesis, showing similar characteristics to human TB disease, such as caseous necrosis, liquefaction, and cavitary disease.
3.4.1 Strain-Dependent Manifestations of Disease
Different strains of mycobacteria cause different disease manifestations:
- CDC1551 strain: Shows similarities to latent TB infection in humans.
- M. bovis: Creates a progressive TB disease that can lead to death.
3.4.2 Microscopic Granuloma Observation After Experimental Challenge
Following experimental challenge, lesions can be seen as early as 2 weeks post-challenge with increased cellularity within the alveolar spaces. Multiple granulomas are observed with strain HN878 at 4 wpc which consist of scattered lymphocytes, neutrophils, and macrophages.
3.4.3 Healing Mechanisms Observed in Granulomas
At 12–16 wpc in HN878 infection, central necrosis increases, liquefaction can be seen in some granulomas, and cavities can also form. Some granulomas, however, have been observed to reduce in size and become mineralized.
3.5 Ruminants as Tuberculosis Models
Ruminants, particularly cattle and goats, serve as valuable animal models for studying tuberculosis due to their natural susceptibility to tuberculous mycobacteria. These models closely resemble bovine and human TB in terms of immune response and pathological characteristics.
3.5.1 Bovine Tuberculosis: Pulmonary Granulomas
Infected bovines show similar characteristics to Mtb-infected humans. The main features of pulmonary granulomas in bTB are necrosis, mineralization, a fibrous capsule, and the presence of MNGCs. AFB are present in higher numbers in more developed granulomas that show necrosis.
3.5.2 Cellular Composition in Bovine Granulomas
Studies have extensively examined the cellular composition of bovine granulomas:
- CD68+ cells: Most abundant in early stages (I and II), scattered throughout the granuloma; less abundant in later stages (III and IV), located around the rim of the necrotic center.
- B cells: Scattered throughout the granuloma in early stages (I/II), moving to follicle-like satellite nests around the outside of the fibrous capsule in advanced lesions (III/IV).
- CD3+ T cells: Similar distribution to CD68+ cells; scattered throughout the lesion at stage I/II, mainly in the outer rim at stage III/IV; CD4+ T cells are the predominant T cell subtype in all stages.
- Neutrophils: Play an important role in early granuloma formation and are associated with the earliest signs of necrosis.
3.5.3 Caprine Tuberculosis: A Model Resembling Human TB
Goat TB closely resembles bovine and human TB in terms of immune response and pathological characteristics. Granulomas in goats are characterized by central caseous necrosis with varying degrees of mineralization, surrounded by epithelioid macrophages, foamy macrophages, MNGCs, lymphocytes, and a fibrotic capsule.
3.6 Zebrafish as Tuberculosis Models
Zebrafish (Danio rerio) have emerged as a widely used alternative vertebrate animal model for studying mycobacterial disease. They can be infected with Mycobacterium marinum, a close relative of MTBC, which induces a systemic TB-like disease.
3.6.1 Granuloma Formation and Histopathological Features
In both larval and adult zebrafish models, granulomas consistent with those reported in human TB can be found in various organs such as the pancreas, gonads, kidney, liver, and occasionally the brain. These early lesions primarily consist of aggregated macrophages, few recruited neutrophils, epithelioid cells, and MNGCs surrounding mycobacteria.
3.6.2 Zebrafish Embryos and Larvae – In Vivo Imaging
The transparency exhibited during the embryo and larval stages offers a distinctive feature for investigating the initial stages of mycobacterial pathogenesis in vivo. Advanced in vivo real-time imaging techniques, including immunofluorescence, mark leukocytes and macrophages in situ.
3.6.3 Granuloma Staging and Acid-Fast Bacilli
Risalde et al. and López et al. classified the granulomas into four histopathological stages based on their extent and cellular composition:
- Type I: Infiltrate of epithelioid macrophages surrounding scattered mycobacteria, without necrotic areas or fibrous capsules.
- Type II: Partially encapsulated granulomas with a cluster of epithelioid macrophages and initial signs of necrosis.
- Type III: Encapsulated and well-organized granulomas containing regions of partial and complete necrosis, along with the presence of AFB.
- Type IV: Encapsulated granuloma with an extensive necrotic core and high mycobacteria concentration.
3.7 Other Animal Models
Numerous other animal species are susceptible to MTBC bacteria, including wildlife species like badgers and wild boar, which are extensively studied due to their epidemiological importance in bTB. Moreover, novel in vitro models such as lung-on-chips are being developed to study MTBC pathogenesis.
4. Challenges and Future Directions
Despite the invaluable insights gained from animal models of tuberculosis, challenges remain in fully replicating the complexity of human disease. Future research should focus on refining existing models, incorporating new technologies, and developing standardized characterization methods to improve the translational relevance of preclinical studies.
4.1 Establishing the Most Appropriate Tuberculosis Model
Identifying the most suitable model for studying tuberculosis requires careful consideration of the specific research question:
- Human-Like Pathology: NHPs and rabbits are suitable for studying cavitary disease and granuloma formation similar to humans.
- Immunological Tools: Mice offer extensive immunological tools for mechanistic studies.
- Drug Efficacy: Guinea pigs are ideal for evaluating drug efficacy due to their comparable disease progression to humans.
4.2 The Role of Molecular Tools in Pathology
The development of new molecular tools in pathology, such as multiplex staining linked to quantitative analysis or spatial transcriptomics, promises to gather valuable knowledge from animal models of TB and the new generation of alternative models to reduce and replace animals following the 3Rs.
4.3 Why Is Standard Characterization Necessary For Pulmonary Lesions?
Standardized characterization and scoring of pulmonary lesions are crucial for:
- Reproducibility: Ensuring consistent results across different studies and laboratories.
- Comparative Analysis: Facilitating meaningful comparisons between different animal models and human disease.
- Translational Relevance: Improving the translatability of preclinical findings to human clinical trials.
5. Conclusion: Advancing Tuberculosis Research with Comprehensive Resources
Tuberculosis remains a significant global health concern, necessitating continuous research efforts to develop new vaccines and therapeutics. Animal models play a vital role in these efforts, providing insights into disease progression and treatment efficacy that are impossible to obtain from human studies alone. A color atlas of comparative pathology of pulmonary tuberculosis, like the resources provided by COMPARE.EDU.VN, would significantly enhance these research endeavors by standardizing lesion characterization, facilitating comparative analysis, and serving as an invaluable educational tool. By embracing these comprehensive resources, the scientific community can advance its understanding of tuberculosis and accelerate progress toward effective control and prevention strategies.
For more in-depth comparisons and detailed information, visit COMPARE.EDU.VN to make informed decisions.
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
Whatsapp: +1 (626) 555-9090
Website: compare.edu.vn
FAQ: Comparative Pathology of Pulmonary Tuberculosis
1. What is pulmonary tuberculosis?
Pulmonary tuberculosis is an infectious disease caused by Mycobacterium tuberculosis that primarily affects the lungs. It is characterized by the formation of granulomas, which are clusters of immune cells attempting to wall off the infection.
2. Why are animal models important in tuberculosis research?
Animal models are essential because they allow researchers to study the pathogenesis, disease progression, and preclinical evaluation of new therapies and vaccines in a controlled setting. Human studies are limited due to ethical constraints and difficulty in obtaining serial tissue samples.
3. What is a granuloma, and why is it significant in tuberculosis?
A granuloma is a hallmark lesion of tuberculosis, regardless of the species or animal model used. It is a structured aggregate of immune cells, including macrophages, lymphocytes, and other inflammatory cells, that forms in response to the presence of mycobacteria. The granuloma’s primary function is to contain the infection and prevent its spread, although it can also serve as a physical barrier to anti-TB drugs.
4. What are the key histopathological features of human pulmonary granulomas?
Key features include a central region of necrosis (caseum), a surrounding layer of epithelioid cells (CD68+), macrophages, and CD4+ T cells, an outer layer of CD8+ T cells, and sometimes, prominent B cell aggregates.
5. How is Ziehl-Neelsen (ZN) staining used in diagnosing tuberculosis?
ZN staining is a routine technique to detect acid-fast bacilli (AFB) in tissues. The mycobacteria appear bright red against a blue background, making them easily identifiable under a microscope.
6. Why is it challenging to study tuberculosis in humans?
It is challenging due to limited access to infected human tissues, the alteration of pathogenic characteristics by therapy, and ethical constraints on performing invasive procedures.
7. What are the main animal models used in tuberculosis research?
The main animal models include non-human primates (macaques, marmosets), rodents (mice, rats), guinea pigs, rabbits, cattle, goats, and zebrafish. Each model has its advantages and limitations.
8. What are the benefits of using non-human primates (NHPs) as tuberculosis models?
NHPs develop the full range of tuberculosis disease observed in humans, including solid lesions, caseation, calcification, and cavitation. They closely mimic the various manifestations of human tuberculosis.
9. What are the limitations of using mice as tuberculosis models?
Mice tend to develop an acute rather than chronic infection, and their lung granulomas lack the structured and organized appearance observed in human granulomas. Most mouse strains do not develop the necrotic caseous center, which is a primary characteristic of human TB.
10. Why are guinea pigs considered the gold standard for preclinical evaluation of tuberculosis vaccines and therapeutics?
Guinea pigs share many features of tuberculosis disease with humans, including the formation of large pulmonary granulomas with central caseating necrosis, making them ideal for evaluating the efficacy of new interventions.