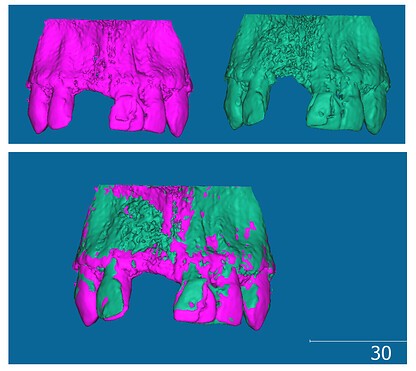
Comparing two CBCT (Cone Beam Computed Tomography) scans is crucial in dental and maxillofacial radiology to quantify changes over time, especially after interventions like bone grafting. This process allows clinicians to objectively assess treatment outcomes by comparing pre-operative and post-operative volumes and densities within a Region of Interest (ROI). This article addresses the challenges and potential workflows for those seeking to Compare Two CBCT volumes using software like 3D Slicer, aiming for accurate quantification of volume and density differences.
One common scenario involves evaluating bone graft volume changes following peri-implant surgery. Clinicians often need to determine the precise volume of bone augmentation achieved and any changes in bone density within the grafted area. To accurately compare two scans acquired at different time points, it’s essential to account for potential discrepancies in patient positioning during each scan. This necessitates robust methods to ensure that the ROI is consistently applied across both datasets.
Currently, a workflow to compare two CBCT scans might involve exporting DICOM images into 3D Slicer, segmenting the region of interest, and then using external software like Autodesk Meshmixer and CloudCompare. This approach, while functional, has limitations. It involves multiple software platforms, increasing complexity and potentially introducing errors during data transfer and conversion. Specifically within 3D Slicer, challenges arise in consistently applying the same ROI across pre-operative and post-operative scans.
3D Slicer offers modules like Registration and ChangeTracker, which are designed to facilitate the comparison of medical images. The Registration module allows for visual alignment of pre-op and post-op scans, enabling a side-by-side comparison. However, it may not directly quantify the volumetric or density differences needed for a comprehensive analysis. The ChangeTracker module, on the other hand, is intended for quantitative change analysis. While it can provide metrics, questions arise regarding the units of measurement (pixels vs. voxels) and the optimal accuracy for volume change assessment when using this module to compare two datasets.
Exploring the Segmentation module within 3D Slicer reveals further complexities. While it provides tabular segmentation statistics, achieving identical ROIs for both pre-operative and post-operative datasets remains a hurdle. Separate segmentations for each scan can introduce variability, making direct comparison less reliable. Incorporating registration techniques within the segmentation workflow is likely necessary to ensure consistent ROI definition when we compare two CBCT volumes.
In conclusion, accurately compare two CBCT scans for volume and density changes requires addressing challenges related to patient positioning discrepancies and consistent ROI application. While 3D Slicer offers various modules that can contribute to this process, a streamlined and robust workflow within the software is still under investigation to achieve reliable and quantitative comparisons. Further exploration of registration and segmentation techniques within 3D Slicer is crucial for developing an effective method to compare two CBCT datasets and precisely quantify post-operative changes.