Molecules are often hailed as the essential building blocks of life, and this statement holds significant truth. Consider water molecules, indispensable for life as we know it on Earth, or protein molecules, crucial for the structure and function of all living organisms. Understanding how atoms, the even more fundamental units, combine to form molecules is therefore a cornerstone in grasping the very origins and nature of life itself. However, the terms “atoms” and “molecules” are frequently used interchangeably or incorrectly, leading to confusion, especially amongst students. This article aims to clarify the key differences between atoms and molecules, providing educators with clear explanations and effective teaching strategies to ensure students develop a solid foundation in chemistry.
What Are Atoms?
Atoms are the most basic units of matter that retain the chemical properties of an element. The concept of atoms dates back to ancient Greece, with Democritus in 400 BC proposing that all matter is composed of indivisible particles he named “atomos.” While our understanding of atomic structure has evolved significantly since then, the fundamental idea remains: atoms are the primary constituents of everything around us.
In the early 19th century, John Dalton provided experimental evidence supporting the atomic theory, envisioning atoms as solid spheres, with different types of spheres representing different elements. Modern science has revealed a more complex picture of atoms, composed of protons, neutrons, and electrons. However, for introductory levels, it’s crucial to establish the atom as the simplest form of an element.
One of the primary challenges in teaching about atoms is their incredibly small size. Atoms are far beyond the realm of direct observation, making it difficult for students to conceptualize them. A practical classroom activity to illustrate the minute size of atoms involves asking students to break down a piece of graphite (pure carbon) into the smallest possible pieces. Despite their efforts, they will never isolate a single carbon atom through physical division. For more advanced students, you could even introduce experiments like the oil film method to estimate the size of a molecule, as detailed by Practical Physics. This hands-on approach helps to bridge the gap between abstract concept and tangible experience.
What Are Molecules?
Molecules are formed when two or more atoms chemically bond together. These atoms can be of the same element, resulting in molecules of an element (like diatomic oxygen, O2, which is essential for respiration, or hydrogen gas, H2). Alternatively, atoms of different elements can combine to form molecules of a compound, such as water (H2O) or carbon dioxide (CO2). The formation of molecules from atoms is driven by the tendency of atoms to achieve a more stable electron configuration.
Understanding molecules is critical because most substances we encounter in everyday life exist as molecules, not as isolated atoms. From the air we breathe (a mixture of molecules like N2 and O2) to the food we eat (complex molecules like carbohydrates and proteins), molecules define the properties and behavior of matter. Teaching about molecules naturally follows the introduction of atoms, emphasizing the process of atoms joining together through chemical bonds.
Key Differences Between Atoms and Molecules
While both atoms and molecules are particles that constitute matter, they exhibit fundamental differences:
| Feature | Atoms | Molecules |
|---|---|---|
| Definition | The smallest unit of an element | Two or more atoms chemically bonded together |
| Stability | Can be stable (noble gases) or unstable | Generally more stable than individual atoms |
| Composition | Single atom | Composed of multiple atoms |
| Charge | Neutral | Neutral |
| Properties | Define the properties of an element | Define the properties of a compound or element |
| Examples | Helium (He), Neon (Ne), Carbon (C) | Water (H2O), Oxygen (O2), Methane (CH4) |

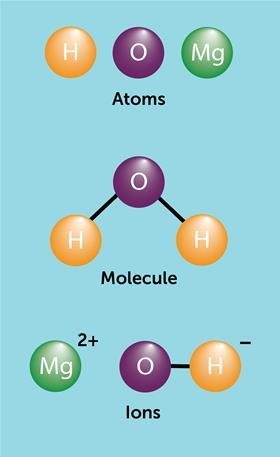
It’s important to note that ions, another type of particle, are distinct from both atoms and molecules. Ions are charged particles formed when atoms or molecules gain or lose electrons. While this article primarily focuses on comparing atoms and molecules, understanding the differences between atoms, molecules, and ions is crucial for a comprehensive grasp of basic chemistry.
Teaching Atoms and Molecules Effectively
Visual aids are invaluable tools for teaching abstract concepts like atoms and molecules. Particle diagrams, which use circles or spheres to represent atoms, can effectively illustrate the difference between individual atoms, molecules of elements, and molecules of compounds. Even Dalton himself utilized diagrams to represent elements and compounds, demonstrating the enduring value of this visual approach. Using different colors to distinguish between different types of atoms further enhances understanding.
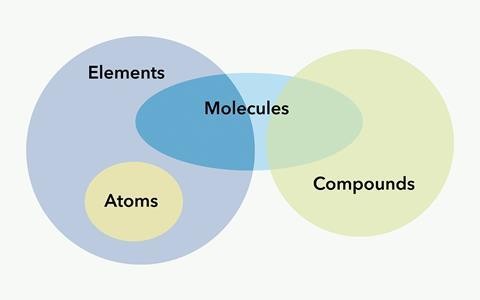
Venn diagrams can also be employed to help students organize their knowledge and visualize the relationships between atoms, elements, molecules, and compounds. Resources like “Atoms, elements, molecules, compounds and mixtures” from the Royal Society of Chemistry provide excellent guidance on using Venn diagrams for this purpose.
Hands-on models are another powerful teaching tool. Molecular model kits allow students to physically construct molecules, making the concept of atoms bonding together more concrete. Games and competitive activities can also inject variety and engagement into lessons. For instance, a game based on “Connect 4,” adapted to atoms, molecules, and ions, can reinforce learning in a fun and interactive way. Downloadable instructions and game cards for such activities are often available from educational resources.
Addressing Common Misconceptions
Students often develop misconceptions regarding atoms and molecules. A common error is referring to ionic compounds as molecules. It is crucial to emphasize that molecules are neutral entities with a defined number of atoms bonded together. In contrast, ionic compounds are composed of a lattice of ions in a fixed ratio, not discrete molecules. Using hands-on models, like TIMSTAR MO84200 for molecules and Molymod MKO-127-27 for ionic structures, can help differentiate these concepts.
Another misconception is the idea that atoms possess the same properties as the bulk material they constitute. For example, students might think a single atom of gold is shiny and yellow, like a gold bar. Similarly, they may incorrectly believe that molecules change their properties in different states of matter (solid, liquid, gas). Addressing these misconceptions explicitly is essential for accurate understanding. Resources like “Beyond appearances: Students misconceptions about basic chemical ideas” offer further insights into common student misunderstandings.
Progression to Advanced Levels
As students progress to higher levels (ages 14-16 and beyond), their understanding of atoms and molecules deepens. They are introduced to subatomic particles (protons, neutrons, electrons) and how these particles define the properties of atoms and ions. They also learn about the different types of forces that govern interactions between atoms, molecules, and ions, which explains the physical properties of ionic and covalent compounds. Resources like “Why do atoms form ions?” are valuable for assessing and reinforcing student understanding at these advanced levels.
Take-Home Points
- Atoms and molecules are fundamental particles of matter, but they are distinct entities.
- Atoms are the basic building blocks of elements, while molecules are formed by the chemical bonding of two or more atoms.
- Clear and consistent language is crucial when teaching these concepts to avoid student confusion.
- Utilize visual aids, hands-on models, and interactive activities to enhance student learning and address common misconceptions.
- A solid understanding of atoms and molecules is foundational for further studies in chemistry, particularly in areas like structure and bonding.
By emphasizing the key differences between atoms and molecules and employing effective teaching strategies, educators can empower students to build a robust understanding of these fundamental concepts, setting them up for success in their future chemistry studies.