Nuclear energy is a powerful force, derived from the very heart of atoms. This energy is harnessed through nuclear reactions, primarily fission and fusion. Both processes alter atoms to release energy, but they operate in fundamentally different ways. Let’s delve into the key differences when we Compare And Contrast Fission And Fusion.
Fission: Splitting Atoms for Energy
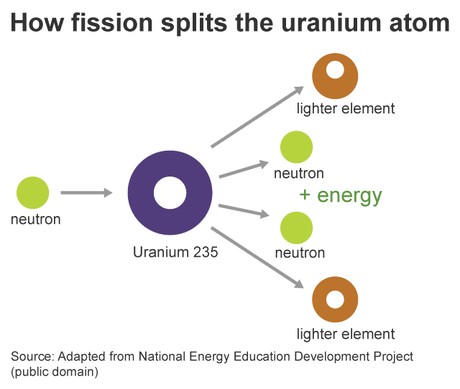
Fission is the process of splitting a heavy, unstable atomic nucleus into two or more lighter nuclei. This “splitting” concept is why it’s named after “binary fission” in biology, referencing cell division. Nuclear fission is typically initiated by bombarding a large isotope with high-speed particles, usually neutrons. Uranium-235 is the most common isotope used in nuclear power plants today.
When a neutron strikes a Uranium-235 nucleus, it becomes unstable and fissions. This fission process yields several products: two smaller isotopes (fission products), multiple high-speed neutrons, and a substantial amount of energy. This released energy is used to heat water, produce steam, and drive turbines to generate electricity in nuclear reactors. Crucially, the ejected high-speed neutrons can then trigger further fission reactions, creating a chain reaction.
Fusion: Joining Atoms for Energy
In contrast to fission, nuclear fusion is the process of combining two light atomic nuclei to form a heavier nucleus. This typically involves isotopes of hydrogen under extreme conditions of pressure and temperature, such as those found in the sun. A common example is the fusion of Deuterium (Hydrogen-2) and Tritium (Hydrogen-3) atoms.
Under immense pressure and heat, these hydrogen isotopes can fuse together, forming a helium isotope and releasing a neutron. This fusion reaction also releases a tremendous amount of energy, significantly more than fission. The sun itself is a giant fusion reactor, where hydrogen atoms are constantly fusing into helium, providing light and heat to our solar system.
Key Differences: Fission vs. Fusion
While both fission and fusion are nuclear processes that generate energy, their differences are significant:
- Process: Fission splits heavy nuclei, while fusion combines light nuclei.
- Elements: Fission typically uses heavy elements like Uranium, while fusion uses light elements like Hydrogen isotopes.
- Conditions: Fission can occur under controlled conditions in nuclear reactors. Fusion requires extreme temperatures and pressures.
- Energy Output: Fusion releases more energy per reaction than fission.
- Radioactive Waste: Fission produces radioactive waste products. Fusion produces significantly less radioactive waste.
- Fuel Source: Uranium for fission is finite. Hydrogen isotopes for fusion are virtually limitless (especially deuterium from seawater).
- Control: Fission is currently controllable and used in power plants. Controlling fusion for sustained energy production is still in the experimental phase.
Conclusion: Distinct Paths to Nuclear Power
In summary, fission and fusion represent two distinct approaches to harnessing nuclear energy. Fission, the splitting of atoms, is a technology already in use for electricity generation. Fusion, the joining of atoms, holds immense potential for clean and abundant energy but presents significant technological challenges. Both fission and fusion play crucial roles in our understanding and utilization of nuclear reactions, shaping the present and future of energy production.