Are Macromolecules Large Or Small Compared To Simple Molecules? COMPARE.EDU.VN helps you understand the scale of life’s building blocks. Discover the vast difference in size between macromolecules and simple molecules, and how this size difference impacts their functions, providing a solution to understanding molecular scales with key insights into biological molecules and their comparative sizes.
1. Introduction: Understanding Molecular Size in Biology
The world of biology operates on a molecular level, where the size of molecules plays a critical role in their function. Macromolecules, the large molecules essential for life, are significantly larger than simple molecules like water or glucose. This difference in size dictates their roles in biological processes. This in-depth comparison, exploring the scale of biological building blocks, enhances your understanding of molecular interactions and functions.
2. Defining Macromolecules and Simple Molecules
2.1 What are Macromolecules?
Macromolecules are large, complex molecules composed of smaller repeating units called monomers. There are four major classes of biological macromolecules: carbohydrates, lipids, proteins, and nucleic acids. These macromolecules are crucial for cell structure, function, and energy storage.
2.2 What are Simple Molecules?
Simple molecules, on the other hand, are small molecules consisting of a few atoms. Examples include water (H2O), carbon dioxide (CO2), glucose (C6H12O6), and amino acids. These molecules serve as the building blocks for macromolecules or play essential roles in cellular processes.
3. Size Comparison: Macromolecules vs. Simple Molecules
3.1 Quantitative Size Differences
Macromolecules are typically thousands to millions of times larger than simple molecules in terms of molecular weight. For example, a single protein molecule can have a molecular weight of 10,000 to 1,000,000 Daltons (Da), while a water molecule weighs only 18 Da. Similarly, a polysaccharide like starch can consist of thousands of glucose molecules.
3.2 Qualitative Size Differences
Qualitatively, the size difference is evident in their physical dimensions and complexity. Simple molecules have simple structures and can easily diffuse through cellular compartments, whereas macromolecules have intricate structures and require specific transport mechanisms.
4. Significance of Size Difference in Biological Functions
4.1 Structural Roles
The large size of macromolecules like proteins and polysaccharides is essential for their structural roles. Proteins form complex three-dimensional structures that provide support and shape to cells and tissues. Polysaccharides like cellulose form the rigid cell walls of plants.
4.2 Catalytic Activity
Enzymes, which are proteins, have specific active sites that bind to substrates (simple molecules) and catalyze biochemical reactions. The large size and complex structure of enzymes allow them to precisely interact with substrates and facilitate reactions.
4.3 Information Storage
Nucleic acids, such as DNA and RNA, store and transmit genetic information. The large size of DNA molecules allows them to encode vast amounts of information, while the smaller size of RNA molecules enables them to carry information from DNA to ribosomes for protein synthesis.
4.4 Energy Storage
Macromolecules like starch and glycogen store large amounts of energy. These polysaccharides are composed of numerous glucose molecules linked together, providing a readily available energy source for cells.
5. Examples of Macromolecules and Simple Molecules in Action
5.1 Proteins: Hemoglobin vs. Amino Acids
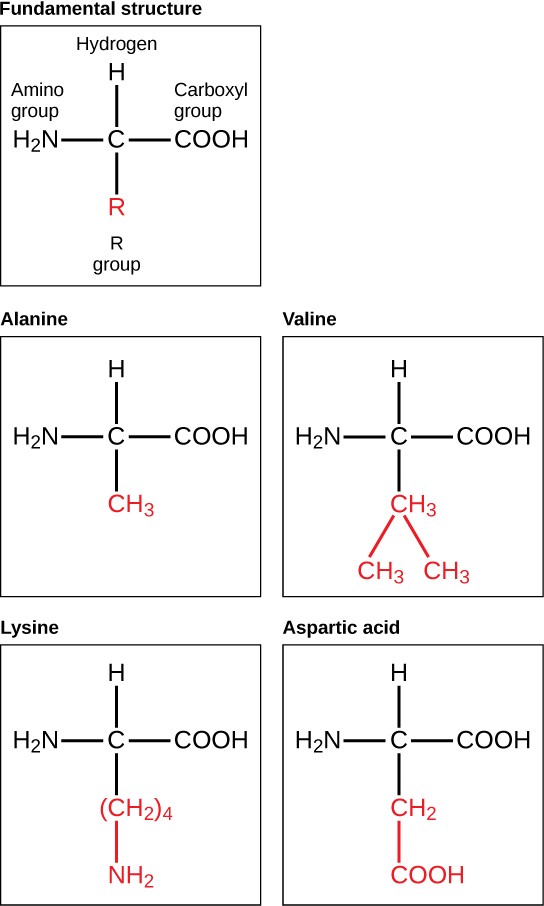
Hemoglobin, a protein found in red blood cells, transports oxygen from the lungs to the tissues. It is composed of four polypeptide chains, each containing hundreds of amino acids. In contrast, amino acids are the simple building blocks of proteins, each consisting of a central carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a variable R group.
5.2 Carbohydrates: Starch vs. Glucose
Starch is a polysaccharide found in plants, serving as a storage form of energy. It consists of thousands of glucose molecules linked together. Glucose, a simple sugar, is a monosaccharide that provides energy to cells through cellular respiration.
5.3 Lipids: Triglycerides vs. Fatty Acids
Triglycerides are fats composed of three fatty acids and glycerol. They store energy and provide insulation. Fatty acids are simple molecules with a long hydrocarbon chain and a carboxyl group.
5.4 Nucleic Acids: DNA vs. Nucleotides
DNA is a nucleic acid that carries genetic information. It is composed of two strands of nucleotides, each containing a nitrogenous base, a pentose sugar, and a phosphate group. Nucleotides are the simple building blocks of DNA and RNA.
6. The Role of Carbon in Forming Macromolecules
6.1 Carbon’s Unique Bonding Properties
Carbon’s ability to form four covalent bonds with other atoms or molecules is fundamental to its role in biological molecules. This bonding versatility allows carbon to create long chains, branching structures, and rings, providing the structural framework for macromolecules.
6.2 Carbon as the Backbone of Organic Molecules
Carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many molecules found uniquely in living things. The simplest organic carbon molecule is methane (CH4), but more complex structures are made using carbon, enabling the diversity of functions of biological macromolecules.
7. Carbohydrates: Energy and Structure
7.1 Monosaccharides, Disaccharides, and Polysaccharides
Carbohydrates are macromolecules represented by the formula (CH2O)n, where n is the number of carbon atoms. They are classified into monosaccharides, disaccharides, and polysaccharides. Monosaccharides are simple sugars like glucose, while disaccharides consist of two monosaccharides, and polysaccharides are long chains of monosaccharides.
7.2 Starch, Glycogen, Cellulose, and Chitin
Starch is the stored form of sugars in plants, while glycogen is the storage form of glucose in animals. Cellulose provides structural support to plant cells, and chitin forms the exoskeleton of arthropods. These polysaccharides demonstrate the diverse functions of carbohydrates through differences in molecular structure.
8. Lipids: Hydrophobic Molecules with Diverse Functions
8.1 Fats, Oils, Waxes, Phospholipids, and Steroids
Lipids are hydrophobic molecules including fats, oils, waxes, phospholipids, and steroids. Fats store energy, phospholipids form cell membranes, and steroids act as hormones. The diversity in lipid structures allows them to perform a wide array of functions in the cell.
8.2 Saturated vs. Unsaturated Fatty Acids
Saturated fatty acids have only single bonds between neighboring carbons, while unsaturated fatty acids have one or more double bonds. Unsaturated fats are liquid at room temperature (oils), while saturated fats are solid (fats). The presence of double bonds affects the packing and properties of fatty acids.
9. Proteins: The Workhorses of the Cell
9.1 Amino Acids and Polypeptides
Proteins are polymers of amino acids, with each amino acid having a central carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a variable R group. Amino acids are linked by peptide bonds to form polypeptides, which then fold into functional proteins.
9.2 Enzymes and Hormones
Proteins function as enzymes, catalyzing biochemical reactions, and as hormones, regulating physiological processes. Enzymes are specific to their substrates, while hormones act as signaling molecules. The diverse functions of proteins underscore their importance in biological systems.
9.3 Primary, Secondary, Tertiary, and Quaternary Structure
Protein structure is organized into four levels: primary (amino acid sequence), secondary (alpha-helices and beta-pleated sheets), tertiary (three-dimensional structure), and quaternary (interaction of multiple polypeptide subunits). The shape of a protein is critical to its function, with changes in temperature, pH, or exposure to chemicals potentially leading to denaturation.
10. Nucleic Acids: Information Storage and Transfer
10.1 DNA and RNA
Nucleic acids, including DNA and RNA, carry the genetic blueprint of the cell and provide instructions for its functioning. DNA stores genetic information, while RNA is involved in protein synthesis. Both are made up of nucleotides, each containing a nitrogenous base, a pentose sugar, and a phosphate group.
10.2 Nucleotides and the Double-Helical Structure of DNA
DNA has a double-helical structure consisting of two strands of nucleotides bonded to each other at their bases with hydrogen bonds. The bases pair such that adenine (A) pairs with thymine (T), and guanine (G) pairs with cytosine (C). This structure allows DNA to store and transmit genetic information accurately.
11. Key Differences Between Macromolecules and Simple Molecules
11.1 Structural Complexity
Macromolecules exhibit complex structures, including folding and branching, while simple molecules have relatively simple structures.
11.2 Molecular Weight
Macromolecules have high molecular weights, ranging from thousands to millions of Daltons, whereas simple molecules have low molecular weights.
11.3 Biological Functions
Macromolecules perform diverse functions, including structural support, catalysis, information storage, and energy storage, while simple molecules serve as building blocks or participate in cellular processes.
11.4 Formation
Macromolecules are formed through polymerization, where monomers are linked together, while simple molecules are typically formed through simple chemical reactions.
12. Importance of Understanding Molecular Size in Biological Contexts
12.1 Drug Design
Understanding the size and structure of macromolecules is crucial in drug design, allowing for the development of drugs that specifically target macromolecules involved in disease processes.
12.2 Material Science
The properties of macromolecules are exploited in material science to create polymers with specific characteristics, such as strength, flexibility, and biocompatibility.
12.3 Nanotechnology
Macromolecules are used in nanotechnology to create nanoscale devices and structures with specific functions, taking advantage of their size and self-assembling properties.
13. Case Studies: Molecular Size and Function
13.1 Sickle Cell Anemia
Sickle cell anemia results from a single amino acid substitution in the hemoglobin protein, causing a change in the shape of red blood cells. This structural change affects the protein’s ability to carry oxygen efficiently, leading to serious health problems.
13.2 Enzyme Specificity
Enzymes exhibit high specificity for their substrates due to the precise fit between the enzyme’s active site and the substrate’s molecular structure. This specificity is essential for catalyzing specific biochemical reactions.
13.3 Viral Structure
Viruses have a capsid, or protein coat, that protects their genetic material. The size and shape of the capsid are crucial for the virus’s ability to infect host cells and replicate.
14. Common Misconceptions About Molecular Size
14.1 Size Equates to Importance
While macromolecules are essential, simple molecules like water and oxygen are equally important for life. Size does not always correlate with importance.
14.2 All Macromolecules Are the Same Size
Macromolecules vary in size depending on their composition and function. Proteins can range from small peptides to large multi-subunit complexes.
14.3 Simple Molecules Are Insignificant
Simple molecules play crucial roles as building blocks for macromolecules and in cellular processes like respiration and photosynthesis.
15. Practical Applications: Visualizing Molecular Size
15.1 Molecular Visualization Software
Software like PyMOL and Chimera allows scientists to visualize the three-dimensional structures of macromolecules and simple molecules, aiding in understanding their properties and interactions.
15.2 Electron Microscopy
Electron microscopy provides high-resolution images of macromolecules, enabling scientists to study their structure and organization at the nanoscale.
15.3 Molecular Modeling
Molecular modeling techniques are used to simulate the behavior of molecules, providing insights into their dynamics and interactions.
16. Future Trends in Molecular Biology
16.1 Advances in Microscopy
Advancements in microscopy techniques, such as cryo-electron microscopy, are allowing scientists to visualize macromolecules at atomic resolution.
16.2 High-Throughput Screening
High-throughput screening methods are used to identify molecules that interact with macromolecules, accelerating drug discovery and materials science research.
16.3 Synthetic Biology
Synthetic biology aims to design and construct new biological systems, including macromolecules, with specific functions.
17. The Impact of Molecular Size on Cellular Processes
17.1 Diffusion and Transport
The size of molecules affects their ability to diffuse through cellular compartments and across membranes. Macromolecules often require specific transport mechanisms, while simple molecules can diffuse more easily.
17.2 Interaction with Cellular Machinery
Macromolecules interact with cellular machinery, such as ribosomes and enzymes, to carry out their functions. The size and shape of these molecules are crucial for these interactions.
17.3 Spatial Organization
The size of macromolecules influences the spatial organization of cellular components, such as organelles and the cytoskeleton.
18. How COMPARE.EDU.VN Can Help You Understand Molecular Comparisons
COMPARE.EDU.VN provides detailed comparisons of biological concepts, including molecular sizes and functions. By using our platform, you can gain a comprehensive understanding of the differences between macromolecules and simple molecules, enabling you to make informed decisions in your studies and professional endeavors. We offer easy-to-understand guides, expert analysis, and user-friendly tools to help you navigate the complexities of molecular biology.
19. Conclusion: Embracing the Molecular World
Understanding the size differences between macromolecules and simple molecules is essential for comprehending biological functions and processes. From structural support to information storage and energy production, the scale of these molecules plays a crucial role in the intricate mechanisms of life. With resources like COMPARE.EDU.VN, you can deepen your knowledge and make informed decisions in your exploration of the molecular world.
20. Call to Action
Ready to dive deeper into the world of molecular biology? Visit COMPARE.EDU.VN today for comprehensive comparisons and expert insights that will help you master complex biological concepts. Whether you’re a student, a researcher, or simply curious, our platform offers the tools and information you need to succeed. Don’t stay puzzled by molecular scales; discover the clarity and knowledge waiting for you at COMPARE.EDU.VN. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States or Whatsapp: +1 (626) 555-9090.
FAQ
1. What are the four major classes of biological macromolecules?
The four major classes of biological macromolecules are carbohydrates, lipids, proteins, and nucleic acids.
2. How do macromolecules differ from simple molecules in size?
Macromolecules are significantly larger than simple molecules, often thousands to millions of times larger in terms of molecular weight.
3. Why is carbon important in the formation of macromolecules?
Carbon’s ability to form four covalent bonds allows it to create long chains, branching structures, and rings, providing the structural framework for macromolecules.
4. What are the main functions of carbohydrates in the body?
Carbohydrates provide energy to the body, particularly through glucose. They also have structural functions, such as cellulose in plant cell walls.
5. What are the different types of lipids, and what are their functions?
Lipids include fats, oils, waxes, phospholipids, and steroids. Fats store energy, phospholipids form cell membranes, and steroids act as hormones.
6. How do proteins perform so many different functions in the cell?
Proteins can function as enzymes, hormones, structural components, and more. Their diverse functions are due to their unique amino acid sequences and three-dimensional structures.
7. What are the four levels of protein structure?
The four levels of protein structure are primary, secondary, tertiary, and quaternary.
8. What are the two main types of nucleic acids, and what are their roles?
The two main types of nucleic acids are DNA and RNA. DNA stores genetic information, while RNA is involved in protein synthesis.
9. How does the size of a molecule affect its ability to move within a cell?
Smaller molecules can diffuse more easily through cellular compartments, while larger molecules may require specific transport mechanisms.
10. How can COMPARE.EDU.VN help me understand molecular comparisons?
compare.edu.vn provides detailed comparisons of biological concepts, including molecular sizes and functions, offering easy-to-understand guides, expert analysis, and user-friendly tools.