Comparative studies often analyze differences between groups, raising the question: Are Comparative Studies Experimental? This analysis delves into a specific comparative study focusing on acute respiratory infection (ARI) treatment to explore this question. The study compares outcomes between a control group and an intervention group utilizing a clinical decision support (CDS) tool.
Comparative Study Design and Methodology
This study employed a comparative design to assess the impact of a CDS tool on ARI management in a primary care setting. Researchers analyzed data from a US primary care research network using a common electronic medical record (EMR) system.
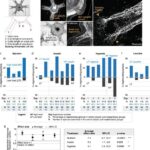
Participants: The study involved two groups: an intervention group of nine practices across nine states and a control group of 61 practices. This multi-state approach enhances the generalizability of the findings.
Intervention: The intervention group received a point-of-care CDS tool integrated into their EMR as customizable progress note templates. The CDS recommendations adhered to Center for Disease Control and Prevention (CDC) guidelines based on patient age and presenting symptoms. The tool aided in ARI diagnosis, antibiotic prescribing decisions, documentation, and access to patient education resources.
Control: The control group continued their standard practices without access to the CDS tool or related training. This allows for a clear comparison of outcomes between the two groups.
Analysis: Data was analyzed quarterly, weighted by the number of ARI episodes to account for varying practice sizes and seasonal fluctuations. Statistical methods included weighted t-tests and linear mixed models to assess differences between groups over time, adjusting for practice characteristics.
Findings and Implications: Are Comparative Studies Experimental?
The study found significant differences between the intervention and control groups. The CDS tool demonstrated a modest reduction in inappropriate antibiotic prescribing for adults and a substantial reduction in broad-spectrum antibiotic use for both adults and children. These findings suggest that the introduction of the CDS tool had a positive impact on prescribing practices. While this comparative study is not a randomized controlled trial (RCT), often considered the gold standard for experimental research, it offers valuable insights into the potential benefits of CDS tools. By carefully controlling for confounding variables and employing rigorous statistical analysis, comparative studies like this can provide strong evidence suggesting causal relationships, even in the absence of random assignment. Therefore, while not strictly experimental, well-designed comparative studies can contribute meaningfully to our understanding of interventions and their effects.
Conclusion
This comparative study demonstrates the potential of CDS tools to improve ARI management. The findings highlight the importance of data-driven decision-making in healthcare and suggest that further research into the implementation and effectiveness of CDS tools is warranted. The study’s rigorous methodology provides compelling evidence, contributing to the ongoing discussion of whether comparative studies can offer insights similar to experimental research.