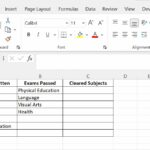
A Student Was Comparing The Solubility Of Equal Amounts of different substances in water, a fundamental concept in chemistry and essential for various applications. At COMPARE.EDU.VN, we provide comprehensive comparisons to help you understand the nuances of solubility and its impact on everyday life. Explore the distinctions between solutions and mixtures, and discover how ionic compounds interact with water using precise analyses.
1. Understanding Solubility: The Foundation of Chemical Interactions
Solubility, at its core, is the measure of how well a solute (a substance being dissolved) dissolves in a solvent (the substance doing the dissolving). Typically, the solvent is a liquid, such as water, and the solute can be a solid, liquid, or gas. The degree to which a substance dissolves is influenced by several factors, including the nature of the solute and solvent, temperature, and pressure. When a substance dissolves, it breaks down into individual molecules or ions and disperses evenly throughout the solvent, forming a homogeneous mixture known as a solution. Understanding solubility is crucial in many fields, from chemistry and biology to environmental science and medicine. It dictates how medications are absorbed in the body, how pollutants spread in water systems, and how chemical reactions occur in solutions. Solubility also plays a key role in industrial processes such as drug manufacturing, food processing, and the production of various chemical compounds. It’s a fundamental property that enables scientists and engineers to control and manipulate chemical systems to achieve desired outcomes. When a student was comparing the solubility of equal amounts, they were essentially investigating how these factors interact to determine the extent to which each substance dissolves.
2. Factors Influencing Solubility: A Detailed Overview
2.1. Nature of Solute and Solvent: Like Dissolves Like
The chemical properties of the solute and solvent are primary determinants of solubility. The general rule “like dissolves like” is particularly useful here. This principle states that polar solvents (those with uneven charge distribution) tend to dissolve polar solutes, while nonpolar solvents (those with even charge distribution) dissolve nonpolar solutes. For example, water is a polar solvent and readily dissolves polar substances like sugar and ionic compounds like salt. Nonpolar solvents, such as oil, are better at dissolving nonpolar substances like fats and waxes. The polarity of a substance is determined by the arrangement of its atoms and the electronegativity differences between them. Polar molecules have a positive end and a negative end, creating a dipole moment that interacts favorably with other polar molecules or ions. Nonpolar molecules, on the other hand, have an even distribution of charge and interact through weaker forces like London dispersion forces. The strength and type of intermolecular forces between solute and solvent molecules play a crucial role in solubility. When the attractive forces between the solute and solvent molecules are stronger than the attractive forces within the solute or solvent alone, the solute is more likely to dissolve.
2.2. Temperature: Heat It Up or Cool It Down
Temperature significantly affects the solubility of most substances. For solids dissolving in liquids, solubility typically increases with temperature. This is because higher temperatures provide more kinetic energy to the molecules, which helps to break the intermolecular forces holding the solid together and allows it to disperse more easily in the solvent. For example, more sugar can dissolve in hot water than in cold water. However, the effect of temperature on the solubility of gases in liquids is the opposite. The solubility of gases decreases as temperature increases. This is because the kinetic energy of the gas molecules increases, allowing them to overcome the attractive forces of the solvent and escape from the solution. This is why carbonated beverages lose their fizz more quickly at room temperature than when chilled. The relationship between temperature and solubility is not always linear and can depend on the specific solute and solvent. Some substances may show a dramatic increase in solubility with temperature, while others may show only a slight change.
2.3. Pressure: A Force to Consider
Pressure has a significant effect on the solubility of gases in liquids but has little to no effect on the solubility of solids or liquids in liquids. According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This means that increasing the pressure of a gas above a liquid will increase the amount of gas that dissolves in the liquid. This principle is used in the production of carbonated beverages, where carbon dioxide is dissolved in the liquid under high pressure. When the pressure is released, such as when opening a bottle of soda, the solubility of the carbon dioxide decreases, and the gas escapes, forming bubbles. Pressure does not significantly affect the solubility of solids or liquids because they are much less compressible than gases. The intermolecular forces holding solids and liquids together are strong enough that changes in pressure do not significantly alter their ability to dissolve.
2.4. Common Ion Effect: Reducing Solubility
The common ion effect refers to the decrease in solubility of an ionic compound when a soluble salt containing a common ion is added to the solution. This effect is a consequence of Le Chatelier’s principle, which states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. In the case of solubility, the stress is the addition of a common ion, which shifts the equilibrium towards the formation of the solid, thus reducing its solubility. For example, the solubility of silver chloride (AgCl) in water is decreased when sodium chloride (NaCl) is added to the solution because both compounds contain the chloride ion (Cl-). The presence of additional chloride ions shifts the equilibrium towards the precipitation of AgCl, reducing the concentration of silver ions (Ag+) in the solution and therefore decreasing the solubility of AgCl. The common ion effect is important in various applications, such as controlling the precipitation of sparingly soluble salts and in analytical chemistry for the quantitative determination of ions in solution.
3. Ionic Substances and Solubility: The Case of Calcium Carbonate and Sodium Bicarbonate
3.1. Ionic Compounds: A World of Charges
Ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). These compounds typically form crystalline lattices, where ions are arranged in a repeating pattern to maximize attractive forces and minimize repulsive forces. The strength of the ionic bonds within the lattice depends on the charges of the ions and the distance between them. Higher charges and shorter distances result in stronger bonds. When an ionic compound dissolves in water, the water molecules must overcome the electrostatic forces holding the ions together in the lattice. Water is a polar solvent, and its molecules can interact with ions through ion-dipole interactions. The positive end of the water molecule (hydrogen) is attracted to anions, while the negative end (oxygen) is attracted to cations. If the ion-dipole interactions are strong enough to overcome the lattice energy of the ionic compound, the ions will be pulled apart and dispersed throughout the water, forming a solution.
3.2. Calcium Carbonate: An Insoluble Exception
Calcium carbonate (CaCO3) is an ionic compound that is notoriously insoluble in water. This insolubility is due to the strong electrostatic forces between the calcium ions (Ca2+) and the carbonate ions (CO32-) in the crystal lattice. These strong forces result in a high lattice energy, which means that a significant amount of energy is required to break the ionic bonds and separate the ions. While water molecules can interact with the calcium and carbonate ions, the ion-dipole interactions are not strong enough to overcome the lattice energy. As a result, calcium carbonate remains largely undissolved in water. The insolubility of calcium carbonate is important in many natural and industrial contexts. It is the primary component of seashells, limestone, and marble, providing structural support for marine organisms and forming geological formations. In industry, calcium carbonate is used as a filler in paper, plastics, and paints, and as an antacid in medications.
3.3. Sodium Bicarbonate: A Soluble Contrast
Sodium bicarbonate (NaHCO3), also known as baking soda, is another ionic compound, but it is much more soluble in water than calcium carbonate. The difference in solubility is due to the weaker electrostatic forces between the sodium ions (Na+) and the bicarbonate ions (HCO3-) in the crystal lattice. These weaker forces result in a lower lattice energy compared to calcium carbonate. When sodium bicarbonate is added to water, the water molecules can effectively interact with the sodium and bicarbonate ions, overcoming the lattice energy and separating the ions. The sodium and bicarbonate ions then disperse throughout the water, forming a solution. The solubility of sodium bicarbonate makes it useful in a variety of applications. It is used as a leavening agent in baking, as an antacid to neutralize stomach acid, and as a cleaning agent. It is also used in fire extinguishers to release carbon dioxide, which smothers the flames.
3.4. Comparing Calcium Carbonate and Sodium Bicarbonate: Why the Difference?
The contrasting solubilities of calcium carbonate and sodium bicarbonate highlight the importance of lattice energy in determining the solubility of ionic compounds. Calcium carbonate has a higher lattice energy due to the higher charge of the calcium ion (Ca2+) compared to the sodium ion (Na+) in sodium bicarbonate. The higher charge results in stronger electrostatic forces and a more stable crystal lattice. Additionally, the carbonate ion (CO32-) is larger and more highly charged than the bicarbonate ion (HCO3-), which also contributes to the higher lattice energy of calcium carbonate. The greater the lattice energy, the more difficult it is for water molecules to break apart the ionic bonds and dissolve the compound. Sodium bicarbonate, with its lower lattice energy, is more easily dissolved in water. In a student’s comparison of the solubility of equal amounts of these two substances, the difference in solubility would be readily apparent. The sodium bicarbonate would dissolve to a much greater extent than the calcium carbonate, demonstrating the impact of lattice energy on solubility.
4. Solutions vs. Mixtures: Understanding the Differences
4.1. Solutions: Homogeneous Harmony
A solution is a homogeneous mixture, meaning that the components are evenly distributed throughout the mixture at a molecular level. In a solution, the solute is completely dissolved in the solvent, and the mixture appears uniform throughout. The dissolved solute particles are so small that they cannot be seen with the naked eye or filtered out using ordinary filter paper. Solutions can be formed from various combinations of solids, liquids, and gases. Examples of solutions include saltwater (salt dissolved in water), sugar water (sugar dissolved in water), and air (a mixture of gases). The properties of a solution are determined by the nature of the solute and solvent and their relative concentrations. Solutions exhibit properties such as clarity, stability, and the ability to conduct electricity if the solute is an electrolyte (a substance that forms ions when dissolved in water).
4.2. Mixtures: Heterogeneous Hodgepodge
A mixture, on the other hand, is a combination of two or more substances that are physically combined but not chemically bonded. Mixtures can be either homogeneous or heterogeneous. In a homogeneous mixture, the components are evenly distributed, but unlike a solution, the components are not dissolved at a molecular level. An example of a homogeneous mixture is milk, which contains fats, proteins, and other substances evenly dispersed in water. In a heterogeneous mixture, the components are not evenly distributed and can be easily distinguished. Examples of heterogeneous mixtures include sand and water, oil and water, and a salad. The components of a heterogeneous mixture can be separated by physical means, such as filtration, decantation, or evaporation.
4.3. Identifying Solutions and Mixtures: Key Characteristics
The key difference between a solution and a mixture lies in the degree of mixing at the molecular level. In a solution, the solute is completely dissolved and dispersed throughout the solvent, forming a homogeneous mixture with uniform properties. In a mixture, the components are physically combined but not chemically bonded, and the mixture may be either homogeneous or heterogeneous. To distinguish between a solution and a mixture, several characteristics can be examined. Solutions are typically clear and transparent, while mixtures may be cloudy or opaque. Solutions do not exhibit the Tyndall effect (the scattering of light by particles in the mixture), while mixtures may show this effect. The components of a solution cannot be separated by filtration, while the components of a mixture can be separated by physical means. A student comparing the solubility of equal amounts of calcium carbonate and sodium bicarbonate would observe that the sodium bicarbonate forms a solution, while the calcium carbonate forms a mixture. The sodium bicarbonate solution is clear and cannot be filtered, while the calcium carbonate mixture is cloudy and can be separated by filtration.
5. Molecular Level Interactions: A Deeper Dive
5.1. Ion-Dipole Interactions: The Driving Force Behind Dissolution
Ion-dipole interactions are attractive forces that occur between ions and polar molecules. These interactions play a crucial role in the dissolution of ionic compounds in polar solvents like water. Water molecules are polar because the oxygen atom is more electronegative than the hydrogen atoms, resulting in an uneven distribution of charge. The oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). When an ionic compound is added to water, the water molecules surround the ions and interact with them through ion-dipole interactions. The positive ends of the water molecules (hydrogen atoms) are attracted to anions, while the negative ends of the water molecules (oxygen atoms) are attracted to cations. These interactions help to stabilize the ions in solution and overcome the lattice energy of the ionic compound. The strength of the ion-dipole interactions depends on the charge of the ion and the dipole moment of the water molecule. Higher charges and larger dipole moments result in stronger interactions. If the ion-dipole interactions are strong enough to overcome the lattice energy, the ionic compound will dissolve in water.
5.2. Lattice Energy: The Barrier to Overcome
Lattice energy is the energy required to separate one mole of an ionic compound into its gaseous ions. It is a measure of the strength of the ionic bonds within the crystal lattice. Higher lattice energies indicate stronger ionic bonds and a more stable crystal lattice. The lattice energy of an ionic compound depends on the charges of the ions, the distance between the ions, and the crystal structure. Higher charges and shorter distances result in higher lattice energies. The crystal structure also affects the lattice energy, with more efficient packing arrangements resulting in higher energies. When an ionic compound dissolves in water, the water molecules must provide enough energy to overcome the lattice energy and separate the ions. If the lattice energy is too high, the water molecules will not be able to break the ionic bonds, and the compound will remain insoluble.
5.3. Hydration: Stabilizing Ions in Solution
Hydration is the process by which water molecules surround and stabilize ions in solution. When an ion is hydrated, it is surrounded by a shell of water molecules that are oriented with their positive or negative ends pointing towards the ion, depending on its charge. This shell of water molecules helps to shield the ion from other ions and to stabilize it in solution. The hydration of ions releases energy, known as the hydration energy, which contributes to the overall energy balance of the dissolution process. Higher hydration energies favor the dissolution of ionic compounds. The hydration energy depends on the charge and size of the ion. Higher charges and smaller sizes result in higher hydration energies. The hydration of ions is a dynamic process, with water molecules constantly exchanging in and out of the hydration shell. This dynamic equilibrium ensures that the ions remain solvated and stable in solution.
6. Polyatomic Ions: Expanding the Ionic Landscape
6.1. What are Polyatomic Ions?
Polyatomic ions are ions composed of two or more atoms that are covalently bonded together and carry an overall charge. Unlike monatomic ions, which consist of a single atom that has gained or lost electrons, polyatomic ions act as a single unit with a net charge. These ions are commonly found in ionic compounds and play important roles in chemical reactions and biological processes. Polyatomic ions can be positively charged (cations) or negatively charged (anions). Examples of common polyatomic cations include ammonium (NH4+) and hydronium (H3O+). Examples of common polyatomic anions include sulfate (SO42-), nitrate (NO3-), phosphate (PO43-), and hydroxide (OH-). The atoms within a polyatomic ion are held together by covalent bonds, which involve the sharing of electrons between atoms. The overall charge of the ion is determined by the balance between the number of protons and electrons in the ion.
6.2. Bicarbonate and Sulfate: Common Examples
The bicarbonate ion (HCO3-) and the sulfate ion (SO42-) are two common polyatomic ions that are important in various chemical and biological contexts. The bicarbonate ion is a negatively charged ion composed of one hydrogen atom, one carbon atom, and three oxygen atoms. It is formed when carbonic acid (H2CO3) loses a proton (H+). The bicarbonate ion plays a crucial role in maintaining the pH balance in blood and other biological fluids. It also acts as a buffer, resisting changes in pH when acids or bases are added. The sulfate ion is a negatively charged ion composed of one sulfur atom and four oxygen atoms. It carries a charge of -2. The sulfate ion is found in many ionic compounds, such as magnesium sulfate (MgSO4), also known as Epsom salt, and potassium sulfate (K2SO4), a common fertilizer. Sulfate ions are also important in biological systems, where they play roles in protein structure, enzyme function, and detoxification processes.
6.3. Role in Solubility: Influencing Interactions
Polyatomic ions influence the solubility of ionic compounds in a similar way to monatomic ions. The charge and size of the polyatomic ion affect the lattice energy of the ionic compound and its interactions with water molecules. Ionic compounds containing polyatomic ions tend to be more soluble in water than those containing only monatomic ions. This is because the larger size and more complex structure of polyatomic ions can weaken the electrostatic forces within the crystal lattice, making it easier for water molecules to break apart the ionic bonds. Additionally, polyatomic ions can form stronger ion-dipole interactions with water molecules, further enhancing their solubility. For example, sodium bicarbonate (NaHCO3), which contains the polyatomic bicarbonate ion, is more soluble in water than calcium carbonate (CaCO3), which contains the polyatomic carbonate ion. The difference in solubility is due to the lower lattice energy of sodium bicarbonate and the stronger ion-dipole interactions between the bicarbonate ion and water molecules.
7. Practical Applications: Solubility in Everyday Life
7.1. Medicine: Drug Delivery and Absorption
Solubility plays a critical role in medicine, particularly in drug delivery and absorption. The solubility of a drug determines how well it dissolves in bodily fluids, such as blood and gastrointestinal fluids, and how easily it can be absorbed into the bloodstream. Drugs that are poorly soluble may have limited bioavailability, meaning that only a small fraction of the drug reaches the target tissues. To improve the solubility of poorly soluble drugs, various techniques are used, such as formulating the drug as a salt, using surfactants to increase its dissolution rate, or encapsulating it in nanoparticles. The solubility of drugs also affects their route of administration. Drugs that are highly soluble can be administered intravenously, allowing for rapid absorption and distribution throughout the body. Drugs that are less soluble may need to be administered orally, with the rate of absorption depending on their dissolution in the gastrointestinal tract.
7.2. Environment: Pollution and Water Quality
Solubility is also important in environmental science, particularly in understanding pollution and water quality. The solubility of pollutants determines how they are transported and dispersed in water systems. Highly soluble pollutants, such as nitrates and phosphates, can easily contaminate groundwater and surface water, leading to eutrophication and other environmental problems. Poorly soluble pollutants, such as heavy metals and organic compounds, may persist in sediments and soils, posing long-term risks to ecosystems. The solubility of pollutants is affected by factors such as pH, temperature, and the presence of other ions in the water. Understanding these factors is crucial for developing effective strategies for pollution control and remediation.
7.3. Industry: Chemical Processes and Manufacturing
In industry, solubility is a key parameter in many chemical processes and manufacturing operations. The solubility of reactants and products affects the rate and yield of chemical reactions. In many cases, reactions are carried out in solution to facilitate the mixing of reactants and to control the reaction rate. The solubility of solids, liquids, and gases in various solvents is also important in separation processes, such as extraction, crystallization, and distillation. These processes are used to purify chemical compounds, recover valuable materials, and remove unwanted impurities. The solubility of materials is also important in the formulation of products such as paints, coatings, and adhesives. The solubility of the various components affects the stability, appearance, and performance of these products.
8. Optimizing Your Understanding of Solubility: Resources at COMPARE.EDU.VN
At COMPARE.EDU.VN, we understand that grasping complex concepts like solubility requires comprehensive resources and clear comparisons. That’s why we offer a range of tools and information to help you deepen your understanding.
8.1. Detailed Comparison Tables
We provide detailed comparison tables that break down the properties of different substances, including their solubility in various solvents. These tables highlight the key factors influencing solubility, such as polarity, temperature, and pressure, allowing you to easily compare and contrast different substances.
8.2. Expert Analyses and Explanations
Our team of experts provides in-depth analyses and explanations of solubility concepts, using clear and concise language to make complex topics accessible to everyone. We explore the underlying principles of solubility, such as ion-dipole interactions, lattice energy, and hydration, providing you with a solid foundation for understanding.
8.3. Real-World Examples and Applications
We illustrate the practical applications of solubility in various fields, from medicine and environmental science to industry and technology. By providing real-world examples, we help you see how solubility impacts your everyday life and the world around you.
8.4. Interactive Quizzes and Assessments
To test your knowledge and reinforce your understanding, we offer interactive quizzes and assessments that cover key solubility concepts. These quizzes provide immediate feedback, allowing you to identify areas where you need to improve and track your progress over time.
9. Troubleshooting Solubility Issues: Common Problems and Solutions
9.1. Poor Solubility of a Desired Compound
One common problem is the poor solubility of a desired compound in a particular solvent. This can be due to various factors, such as the polarity mismatch between the compound and the solvent, the presence of strong intermolecular forces within the compound, or the formation of stable crystal lattices. To address this issue, several strategies can be employed:
-
Change the Solvent: Experiment with different solvents to find one that better matches the polarity of the compound. Polar compounds are more soluble in polar solvents, while nonpolar compounds are more soluble in nonpolar solvents.
-
Modify the Compound: Chemically modify the compound to increase its polarity or reduce its intermolecular forces. This can be achieved by adding polar functional groups or breaking up the crystal lattice.
-
Use a Co-Solvent: Add a small amount of a co-solvent that is miscible with the original solvent and has a higher affinity for the compound. This can help to increase the solubility of the compound in the mixture.
-
Increase the Temperature: Heating the solvent can often increase the solubility of the compound by providing more energy to overcome the intermolecular forces.
9.2. Precipitation of a Compound from Solution
Another common problem is the precipitation of a compound from solution, which can occur when the solution is cooled, evaporated, or when a common ion is added. To prevent precipitation, several strategies can be used:
-
Control the Temperature: Maintain the solution at a constant temperature to prevent changes in solubility.
-
Avoid Evaporation: Keep the solution covered to prevent evaporation of the solvent, which can increase the concentration of the compound and cause it to precipitate.
-
Remove Common Ions: If precipitation is caused by the common ion effect, remove the common ions from the solution by adding a complexing agent or by using ion exchange resins.
-
Add a Stabilizer: Add a stabilizer, such as a polymer or surfactant, to prevent the compound from aggregating and precipitating out of solution.
9.3. Slow Dissolution Rate
Sometimes, a compound may be soluble in a particular solvent, but it dissolves very slowly. This can be due to factors such as the particle size of the compound, the viscosity of the solvent, or the presence of a surface layer that inhibits dissolution. To increase the dissolution rate, the following methods can be used:
-
Reduce Particle Size: Grinding the compound into a fine powder can increase its surface area and speed up the dissolution process.
-
Stir the Solution: Stirring the solution can help to mix the compound with the solvent and prevent the formation of a saturated layer around the particles.
-
Heat the Solution: Heating the solution can increase the kinetic energy of the molecules and speed up the dissolution process.
-
Add a Surfactant: Adding a surfactant can reduce the surface tension of the solvent and promote the wetting of the compound, leading to faster dissolution.
10. Key Takeaways and Future Directions
Solubility is a fundamental property that governs many chemical and biological processes. Understanding the factors that influence solubility, such as the nature of the solute and solvent, temperature, and pressure, is crucial for controlling and manipulating chemical systems to achieve desired outcomes. The contrasting solubilities of ionic compounds like calcium carbonate and sodium bicarbonate highlight the importance of lattice energy and ion-dipole interactions in determining solubility. Solutions and mixtures differ in their degree of mixing at the molecular level, with solutions being homogeneous mixtures where the solute is completely dissolved in the solvent. Polyatomic ions, such as bicarbonate and sulfate, play important roles in solubility and have significant implications in various fields. Practical applications of solubility span medicine, environment, and industry, impacting drug delivery, pollution control, and chemical manufacturing. As we move forward, future research in solubility will focus on developing new techniques for enhancing the solubility of poorly soluble drugs, designing more effective strategies for pollution remediation, and creating innovative materials with tailored solubility properties.
11. Frequently Asked Questions (FAQs) About Solubility
1. What is solubility and why is it important?
Solubility is the ability of a substance (solute) to dissolve in a solvent. It is crucial because it affects chemical reactions, drug delivery, environmental processes, and industrial applications.
2. What factors affect the solubility of a substance?
Key factors include the nature of the solute and solvent (like dissolves like), temperature, pressure (for gases), and the presence of other ions (common ion effect).
3. What is the “like dissolves like” rule?
Polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. This is due to similar intermolecular forces.
4. How does temperature affect solubility?
Generally, the solubility of solids in liquids increases with temperature, while the solubility of gases in liquids decreases with temperature.
5. What is the common ion effect?
The common ion effect is the decrease in solubility of an ionic compound when a soluble salt containing a common ion is added to the solution.
6. What is the difference between a solution and a mixture?
A solution is a homogeneous mixture where the solute is completely dissolved. A mixture can be homogeneous or heterogeneous, with components physically combined but not chemically bonded.
7. What are polyatomic ions, and how do they affect solubility?
Polyatomic ions are ions composed of two or more atoms bonded together. They affect solubility based on their charge, size, and interactions with water molecules.
8. Why is calcium carbonate insoluble in water, while sodium bicarbonate is soluble?
Calcium carbonate has a higher lattice energy due to stronger ionic bonds, making it less soluble. Sodium bicarbonate has a lower lattice energy and forms stronger ion-dipole interactions with water.
9. How is solubility important in medicine?
Solubility affects drug delivery and absorption. Drugs must be soluble to be effectively absorbed into the bloodstream and reach target tissues.
10. How does COMPARE.EDU.VN help with understanding solubility?
COMPARE.EDU.VN provides detailed comparisons, expert analyses, real-world examples, and interactive quizzes to enhance understanding of solubility.
12. Discover More at COMPARE.EDU.VN
Navigating the complexities of solubility and other scientific concepts can be challenging. At COMPARE.EDU.VN, we’re dedicated to providing you with the resources you need to make informed decisions.
Ready to dive deeper and explore more comparisons? Visit COMPARE.EDU.VN today to discover comprehensive analyses and expert insights. Whether you’re a student, a professional, or simply curious, we’re here to help you compare, contrast, and choose with confidence.
Need assistance or have questions?
Contact us at:
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
Whatsapp: +1 (626) 555-9090
Website: COMPARE.EDU.VN
Make the smart choice with compare.edu.vn. Your journey to informed decisions starts here!