Understanding the atom, the fundamental building block of matter, has been a journey of continuous refinement and revision. Scientists have developed various models over time, each building upon previous knowledge and incorporating new discoveries. This article explores how a student might compare different atomic models, highlighting their key features and limitations.
Early Atomic Models: Solid Spheres and Plum Pudding
Initial conceptions of the atom were rudimentary. The Greek philosopher Democritus proposed the idea of indivisible particles called “atomos,” meaning uncuttable. This early model envisioned atoms as solid, indestructible spheres.
Later, in the late 19th and early 20th centuries, J.J. Thomson’s discovery of the electron led to the “plum pudding” model. This model depicted the atom as a positively charged sphere with negatively charged electrons embedded within it, much like plums in a pudding. This model successfully incorporated the newly discovered electron but failed to explain later experimental results.
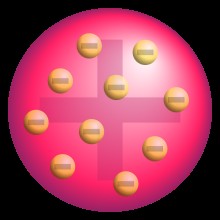 Plum Pudding Model of the atom
Plum Pudding Model of the atom
Rutherford’s Nuclear Model: A Major Leap Forward
Ernest Rutherford’s gold foil experiment revolutionized atomic theory. By bombarding a thin gold foil with alpha particles, Rutherford observed that most particles passed straight through, while a few were deflected at large angles. This led him to propose the nuclear model, where the atom consists of a dense, positively charged nucleus containing most of the atom’s mass, surrounded by orbiting electrons. This model introduced the concept of a mostly empty atom with a concentrated nucleus.
Bohr’s Model: Quantized Orbits and Energy Levels
Niels Bohr further refined Rutherford’s model by incorporating the principles of quantum mechanics. Bohr proposed that electrons orbit the nucleus in specific, quantized energy levels. Electrons could only exist in these defined orbits and could jump between them by absorbing or emitting energy. While Bohr’s model successfully explained the hydrogen atom’s spectrum, it had limitations when applied to more complex atoms.
The Quantum Mechanical Model: Probability and Orbitals
The current understanding of the atom is based on the quantum mechanical model. This model describes electrons not as particles orbiting in fixed paths but as existing in probability clouds called orbitals. Orbitals represent regions where there is a high probability of finding an electron. This model incorporates the wave-particle duality of electrons and provides a more accurate and comprehensive description of atomic structure.
Comparing and Contrasting: A Student’s Perspective
A student comparing these models would note the progressive development in understanding atomic structure. From solid spheres to the complex quantum mechanical model, each model represents a significant step forward. Key differences lie in the description of the electron’s location and behavior. The evolution of these models demonstrates the dynamic nature of scientific knowledge, where new evidence leads to revised and refined understanding. Each model served as a stepping stone, building upon previous limitations and leading to a more comprehensive picture of the atom.
Conclusion: The Ever-Evolving Atom
The models of the atom discussed here illustrate the power of scientific inquiry and the evolution of scientific understanding. As a student delves deeper into the world of atoms, comparing these models provides a valuable framework for understanding the complexity of matter and the scientific process itself. The journey of atomic discovery continues, driven by ongoing research and technological advancements.