A single bond, compared to a double bond, is longer and weaker. This fundamental concept in chemistry explains the relationship between bond order, bond length, and bond energy. Understanding these relationships is crucial for predicting molecular properties and reactivity.
Bond Order, Length, and Energy: The Fundamentals
Bond order refers to the number of electron pairs shared between two atoms. A single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3.
Bond length is the distance between the nuclei of two bonded atoms.
Bond energy (or bond dissociation energy) is the energy required to break a bond between two atoms in the gaseous phase.
The relationship between these three properties is as follows:
- Higher bond order leads to shorter bond length and higher bond energy. A double bond, with its two shared electron pairs, pulls the atoms closer together than a single bond with only one shared pair. This closer proximity results in a stronger attraction and thus requires more energy to break.
- Lower bond order leads to longer bond length and lower bond energy. A single bond, with its greater distance between atoms, experiences a weaker attraction and requires less energy to break.
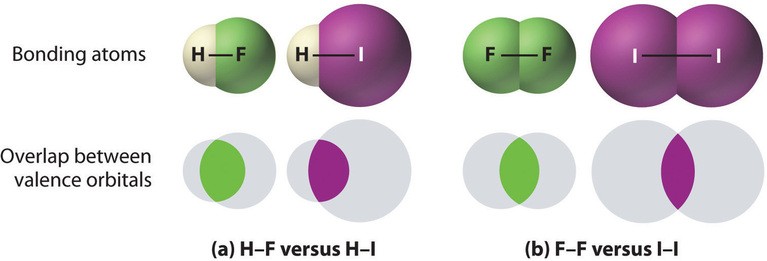
Figure: The strength of covalent bonds depends on the overlap between the valence orbitals of the bonded atoms. Larger atoms have less effective overlap, resulting in weaker bonds.
Comparing Single and Double Bonds: Key Differences
Let’s summarize the key differences between single and double bonds:
| Feature | Single Bond | Double Bond |
|---|---|---|
| Bond Order | 1 | 2 |
| Bond Length | Longer | Shorter |
| Bond Energy | Lower | Higher |
| Reactivity | Generally less reactive | Generally more reactive |
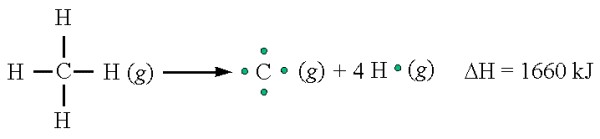
Factors Influencing Bond Energy
While bond order is a primary factor influencing bond energy, other factors also play a role:
- Atom Size: Bonds between smaller atoms are generally stronger due to better orbital overlap. For example, an H-F bond is stronger than an H-I bond.
- Atom Electronegativity: Differences in electronegativity between bonded atoms can influence bond strength. Larger differences can lead to stronger bonds.
- Molecular Environment: The surrounding atoms and bonds in a molecule can subtly influence the strength of a particular bond.
Applying Bond Energy: Estimating Enthalpy Changes
Average bond energies can be used to estimate the enthalpy change (ΔH) of a chemical reaction. This is done by calculating the difference between the energy required to break bonds in the reactants and the energy released when forming bonds in the products:
ΔH ≈ Σ (bond energies of bonds broken) – Σ (bond energies of bonds formed)
Figure: Breaking the C-H bonds in methane requires energy input.
For example, in the combustion of methane (CH4), four C-H bonds and two O=O bonds are broken, while four C=O bonds and four O-H bonds are formed. Using average bond energy values, we can estimate the enthalpy change for this reaction. This method provides a valuable tool for predicting the energy output or input associated with a chemical reaction.
Conclusion
The principle that “A Single Bond Compared To A Double Bond Is Longer” is a cornerstone of chemical bonding theory. Understanding this relationship, along with the associated concepts of bond order and bond energy, is essential for comprehending molecular structure, properties, and reactivity. These concepts are crucial for various applications, including predicting reaction outcomes and designing new materials.