The potential of statins, like lovastatin and simvastatin, to treat cognitive deficits in neurodevelopmental disorders, such as Neurofibromatosis type 1 (NF1), Fragile X syndrome, and autism, has been the subject of extensive research. This analysis delves into the implications of a comparative study (often referencing findings similar to Ottenhoff 1990, though that specific study isn’t directly analyzed here) examining lovastatin and simvastatin’s efficacy in treating these disorders, specifically focusing on a recent preclinical study in a Fragile X mouse model. This study, and others like it, highlight the complexities of translating preclinical findings to human clinical trials.
Lovastatin vs. Simvastatin: Preclinical Findings and Clinical Challenges
Nearly three decades ago, research identified the potential of statins to antagonize RAS (rat sarcoma viral oncogene homolog) signaling, a critical pathway in several neurodevelopmental disorders. Statins, as HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase inhibitors, reduce the production of farnesyl, necessary for RAS activation. This led to investigations into their therapeutic potential in RASopathies, disorders caused by RAS/ERK (extracellular signal-regulated kinase) pathway overactivation.
Lovastatin showed promising results in preclinical models of NF1 and Noonan syndrome, but not in Costello syndrome. Subsequent clinical trials with lovastatin and simvastatin in NF1 patients yielded largely negative results. A recent study suggested simvastatin might benefit children with autism spectrum disorder (ASD) by reducing irritability and hyperactivity.
A pivotal preclinical study compared lovastatin and simvastatin in a Fragile X mouse model (Fmr1 mice). While both statins inhibit HMG-CoA reductase, simvastatin demonstrates higher potency and better blood-brain barrier permeability. Surprisingly, the study concluded that lovastatin was superior to simvastatin in rescuing Fmr1 phenotypes, raising concerns about simvastatin’s suitability for treating neurodevelopmental disorders.
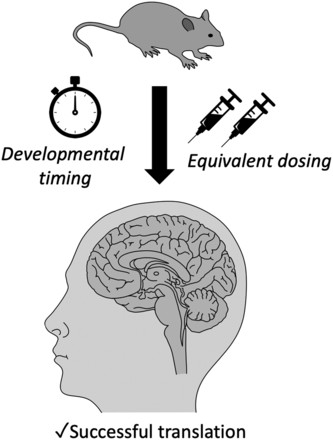 Figure 1.
Figure 1.
Critical Analysis of the Comparative Study
The comparative study’s conclusion warrants careful scrutiny. Several factors raise questions about its methodology and interpretation:
-
Dosage Discrepancies: The study employed significantly different dosages of lovastatin and simvastatin, making direct comparisons challenging. Lovastatin was often used at much higher doses, potentially obscuring simvastatin’s true effects. The study did reveal that lower doses of simvastatin worsened certain Fmr1 phenotypes, a finding with potential clinical implications. However, it remains unclear whether similar low doses of lovastatin would produce the same effect.
-
Lack of Direct Statistical Comparisons: The study lacked direct statistical comparisons between lovastatin and simvastatin at the same dose, hindering a definitive assessment of their relative efficacy. A re-analysis of the audiogenic seizure data, using a logistic regression model to account for differences in vehicle, suggests that lovastatin, even at higher doses, did not perform significantly better than simvastatin.
Translational Challenges: From Mice to Men
Two crucial factors complicate the translation of preclinical findings to human clinical trials:
-
Dose Translation: Doses effective in animal models may not translate to safe and effective doses in humans. The high lovastatin dose used in the Fmr1 study far exceeds clinically used doses, limiting its translational relevance. Even with comparable oral doses, species differences in blood-brain barrier permeability could impact statin efficacy. Further research is needed to determine optimal dosing ranges in humans.
-
Timing of Intervention: Preclinical studies often treat adult animals, while optimal treatment in humans might require early intervention during childhood. Conversely, positive effects observed in young animals need to be verified in adults. Defining the critical period for intervention is essential for successful clinical translation.
Conclusion: Future Directions
The comparative study highlighted by this analysis, alongside other research in the field, underscores the complexity of using statins to treat neurodevelopmental disorders. While preclinical studies offer valuable insights, careful consideration of dosage, timing of intervention, and rigorous methodological approaches are crucial for successful translation to human clinical trials. Future research should focus on:
-
Comparative Dose-Response Studies: Conducting studies with equivalent dosages of lovastatin and simvastatin across a range of concentrations is essential for accurate comparisons.
-
Pharmacokinetic and Pharmacodynamic Studies: Investigating species differences in drug metabolism and brain penetration will improve dose translation from animal models to humans.
-
Critical Period Determination: Identifying the optimal developmental window for statin intervention is crucial for maximizing therapeutic efficacy.
By addressing these challenges, future research can unlock the full therapeutic potential of statins in treating neurodevelopmental disorders.
