Are you seeking a comprehensive comparison between resveratrol and caloric restriction? COMPARE.EDU.VN offers an insightful exploration of these two anti-aging strategies, providing you with a clear understanding of their benefits and differences. By comparing their mechanisms and effects, we equip you with the knowledge to make informed decisions about your health and well-being. Explore diverse comparison resources to gain a comprehensive understanding and informed decision-making, enhancing your strategic decision-making capabilities.
1. Introduction to Anti-Aging Strategies: Resveratrol and Caloric Restriction
In recent years, advancements in healthcare have significantly increased human life expectancy, leading to a growing elderly population. Consequently, age-related diseases have become major health concerns in developed nations. While modifying our genetic makeup remains a distant prospect, various anti-aging strategies are emerging to promote healthier lifestyles and therapeutic interventions. Two of the most promising approaches are resveratrol supplementation and caloric restriction (CR). This comparative study delves into the effects of these strategies, particularly focusing on their impact on the longevity-associated gene silencing information regulator (SIRT1).
1.1 Caloric Restriction: A Time-Tested Method for Longevity
Caloric restriction, involving reduced calorie intake without malnutrition, has consistently demonstrated its ability to extend lifespan in various model organisms, from yeast to primates [2]. CR offers protection against the decline of biological functions, lowering the incidence and delaying the onset of multiple age-related diseases. Its mechanisms involve several key processes:
- Growth Retardation: Slowing down the rate of growth.
- Body Fat Reduction: Decreasing overall body fat.
- Neuroendocrine Changes: Delaying changes in the neuroendocrine system.
- DNA Repair Enhancement: Boosting the body’s ability to repair damaged DNA.
- Gene Expression Alteration: Modifying gene expression patterns.
- Apoptosis Enhancement: Promoting programmed cell death of damaged cells.
- Metabolic Rate Depression: Lowering the metabolic rate.
- Oxidative Stress Amelioration: Reducing oxidative stress.
The anti-aging benefits of CR are strongly associated with increased levels and activation of sirtuins, especially SIRT1. Other involved molecular signaling pathways include peroxisome proliferator activated receptor G coactivator-1α (PGC-1α), adenosine monophosphate activated protein kinase (AMPK), insulin/insulin growth factor-1, and target of rapamycin [1, 4].
1.2 Resveratrol: A Natural Mimetic of Caloric Restriction
Resveratrol, a plant polyphenol found in grapes, berries, and peanuts, has emerged as a potential therapeutic option for anti-aging. Resveratrol’s integration into dietary supplements and foods mirrors the beneficial effects of CR [6], positioning it as a promising CR mimetic [4]. Research indicates that resveratrol extends lifespan by significantly increasing SIRT1 activity, enhancing its affinity for both NAD+ and acetylated substrates [7, 8]. This mechanism is also believed to contribute to the longevity associated with CR.
1.3 Why Compare Resveratrol and Caloric Restriction?
While both resveratrol and CR have been extensively studied for their potential health benefits, a comprehensive comparison of their effects remains limited. This study aims to bridge this gap by comparatively assessing the anti-aging effects of resveratrol and CR, both in vivo and in vitro, with a primary focus on the SIRT1 pathway. We aim to provide a deeper understanding of how these interventions influence cellular senescence, apoptosis, cognitive function, and oxidative stress.
2. Comparative Study Design and Methodology
To effectively compare the anti-aging properties of resveratrol and caloric restriction, the study employed both in vitro and in vivo models. Each model was designed to closely simulate aging processes and allow for measurable comparisons of the interventions.
2.1 In Vitro Model: AAPH-Induced Senescence in IMR-90 Cells
The in vitro component utilized the human diploid fibroblast strain IMR-90, obtained from ATCC, to establish a cellular senescence model. Cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in a 5% CO2 environment. To induce senescence, cells were treated with 1 mM 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) for 48 hours.
- AAPH Treatment: AAPH, a free radical initiator, was used to induce oxidative stress and simulate the cellular damage associated with aging. This method allows for controlled induction of senescence without biotransformations or enzymes [12, 13].
- Intervention Groups: After AAPH treatment, cells were divided into three groups:
- Resveratrol Group: Cells were incubated with culture medium containing resveratrol (5 μM, 10 μM, and 20 μM).
- Caloric Restriction Group: Cells were incubated with MEM supplemented with 3% FBS.
- Control Group: Cells were incubated with standard culture medium alone.
2.2 In Vivo Model: D-Galactose-Induced Aging in Rats
The in vivo component used male albino Wistar rats weighing approximately 200 ± 10 g. These rats were acclimated for two weeks before the experiment at the Experimental Animal Center of Hubei University of Chinese Medicine. The rats were then divided into five groups:
- Negative Control Group (NG): Received the vehicle alone (0.9% saline).
- Model Control Group (MG): Received D-galactose (200 mg/kg bodyweight).
- Low Dose Resveratrol Group (RESL): Received D-galactose and resveratrol (50 mg/kg bodyweight).
- High Dose Resveratrol Group (RESH): Received D-galactose and resveratrol (100 mg/kg bodyweight).
- Caloric Restriction Group (CR): Received D-galactose and caloric restriction (40% lower in calories than ad libitum fed rats [27]).
2.3 Key Parameters Measured
Both in vitro and in vivo models were assessed using various parameters to evaluate the anti-aging effects of resveratrol and CR:
- SA β-gal Staining: Used to detect senescence-associated β-galactosidase (SA β-gal) activity in IMR-90 cells, a marker of cellular senescence [26].
- Scanning Electron Microscopy: Employed to analyze morphological changes in IMR-90 cells.
- Apoptosis Analysis: Measured using PI/Annexin V staining assay to determine phosphatidylserine expression, an indicator of early-stage apoptosis.
- Body Weight Monitoring: Tracked to observe any changes in body weight during the experimental period.
- Morris Water Maze Test: Conducted to assess spatial learning and memory in rats.
- Oxidative Stress Markers: Measured levels of superoxide dismutase (SOD), total antioxidant capacity (T-AOC), malondialdehyde (MDA), and lipofuscin in rat tissues.
- Telomerase Activity: Assessed in liver and brain tissues using telomerase ELISA kits.
- Gene Expression Analysis: Quantitative real-time RT-PCR was used to determine mRNA expression levels of SIRT1, p53, and Foxo3a.
- Protein Expression Analysis: Western blot assays were performed to measure protein levels of p53, FOXO3a, HuR, AROS, and DBC1.
- Immunohistochemistry: Used to analyze FOXO3a and p53 expression in brain and liver tissues.
2.4 Statistical Analysis
Results were expressed as means ± SD and analyzed using SPSS 18.0 software. Group differences were determined using ANOVA followed by post-hoc Tukey test. A P value less than 0.05 was considered significant.
3. Comparative Results: Resveratrol vs. Caloric Restriction
The study yielded several significant findings that highlight the comparative effects of resveratrol and caloric restriction on aging processes.
3.1 In Vitro Results: IMR-90 Cells Treated with AAPH
- SA β-gal Staining: AAPH-treated cells showed significantly more positive staining than control cells. Resveratrol (5 μM, 10 μM, and 20 μM) and CR treatments markedly protected against AAPH-induced senescence. Notably, cells treated with 10 μM resveratrol displayed less SA β-gal positive staining than CR-treated cells, indicating a more efficient inhibition of AAPH-induced senescence.
- Scanning Electron Microscopy: Normal IMR-90 cells appeared fusiform with few blebs, whereas AAPH-treated cells showed an enlarged, flattened morphology with numerous blebs. Resveratrol and CR treatments maintained the normal fusiform shape with fully spread cells, suggesting protection against AAPH-induced morphological changes.
- Apoptosis Analysis: The population of apoptotic cells significantly increased in the AAPH group (34.54%) compared to the negative group (7.32%). Resveratrol and CR treatments reduced apoptosis, with 10 μM resveratrol more efficiently decreasing AAPH-induced apoptosis (11.58%) than CR treatment (16.78%).
3.2 In Vivo Results: D-Galactose-Treated Rats
-
General Appearance and Body Weight: Rats in the D-gal model group exhibited signs of aging, including apathy, dullness, brittle hair, and inelastic skin. Resveratrol and CR treatments reversed these changes. No significant difference in body weight was observed between experimental groups, except for the CR group, which showed almost no weight increase during the experiment.
-
Morris Water Maze Test: The D-gal group showed longer escape latency and lower swim speed compared to the control group, indicating impaired spatial learning and memory. Resveratrol and CR treatments reversed these effects. The high-dose resveratrol group displayed relatively longer escape latency and lower swim speed compared to the CR group.
- Oxidative Stress Markers: D-gal treatment significantly decreased SOD and T-AOC levels and increased MDA levels in serum, liver, and brain. Resveratrol and CR treatments reversed these effects. Administration of high-dose resveratrol showed superior results compared to CR treatment.
Table 1. Antioxidant Status in Tissues and Blood of Rats
| Index | Group | Liver | Brain | Serum |
|---|---|---|---|---|
| MDA (nmol/mg prot or nmol/ml) | NG | 2.96 ± 0.26 | 2.93 ± 0.04 | 11.85 ± 0.44 |
| MG | 3.40 ± 0.32* | 3.51 ± 0.04** | 14.12 ± 0.9* | |
| CR | 3.04 ± 0.60# | 3.05 ± 0.08## | 12.82 ± 1.46 | |
| RESL | 3.18 ± 0.35 | 3.12 ± 0.07*# | 11.97 ± 0.65 | |
| RESH | 2.88 ± 0.65# | 2.83 ± 0.09##∆ | 9.41 ± 1.23# | |
| SOD (U/mg prot or U/ml) | NG | 307.38 ± 24.32 | 323.51 ± 33.17 | 85.50 ± 3.13 |
| MG | 280.38 ± 18.10* | 241.33 ± 46.06** | 78.99 ± 2.33* | |
| CR | 294.19 ± 24.21 | 305.03 ± 53.2# | 81.81 ± 3.38 | |
| RESL | 272.65 ± 13.84 | 300.46 ± 46.59# | 83.39 ± 2.56 | |
| RESH | 305.43 ± 28.61# | 331.92 ± 52.45##∆ | 84.37 ± 2.77# | |
| T-AOC (U/mg prot or U/mL) | NG | 1.13 ± 0.05 | 0.71 ± 0.07 | |
| MG | 0.97 ± 0.06* | 0.57 ± 0.03** | ||
| CR | 1.09 ± 0.04# | 0.64 ± 0.04# | ||
| RESL | 1.06 ± 0.08 | 0.70 ± 0.03## | ||
| RESH | 1.19 ± 0.08#∆ | 0.89 ± 0.08##∆ |
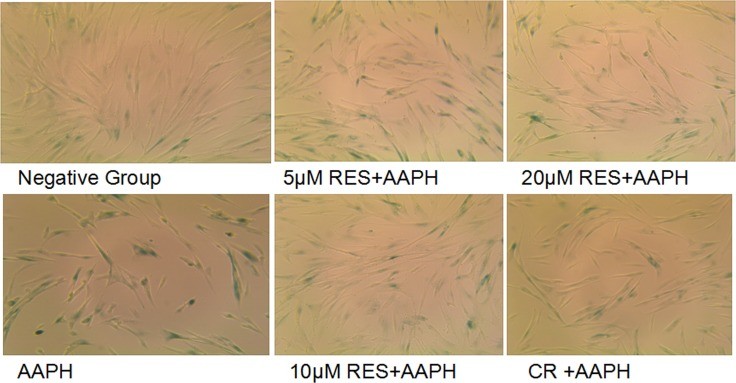
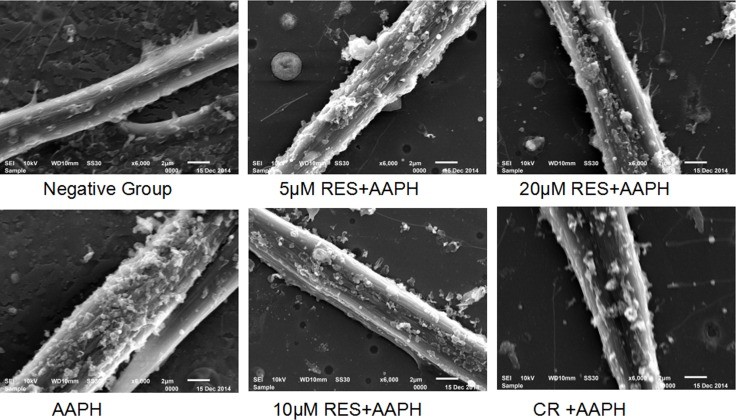
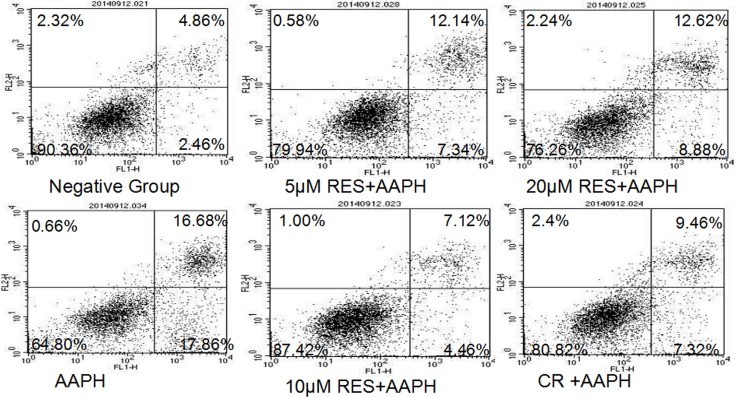
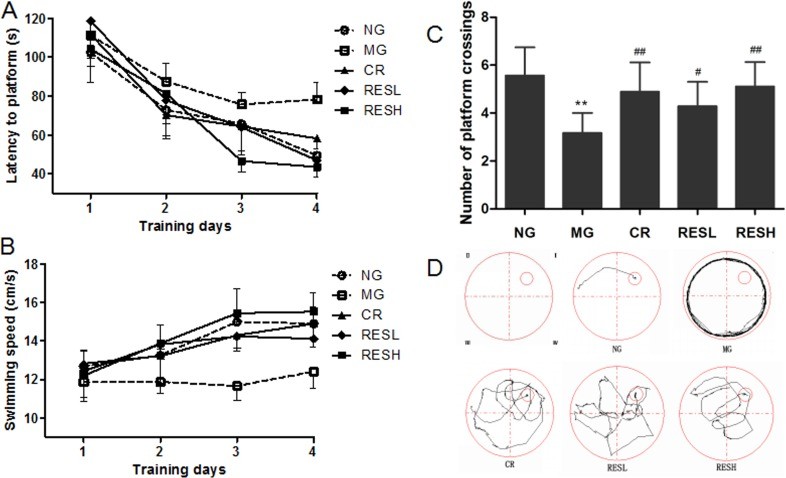
P < 0.05, ** P < 0.01 compared with control group (all groups); # P < 0.05, ## P < 0.01 compared with model group (CR group and RES group); ∆ P < 0.05, ∆∆ P < 0.01 compared with CR group (RES group).
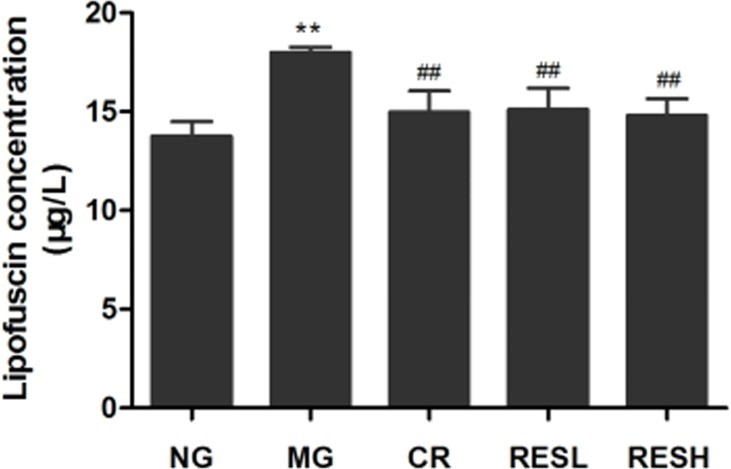
- Lipofuscin Levels: D-gal-treated rats showed a significant increase in lipofuscin levels compared to the control group. Resveratrol and CR treatments decreased lipofuscin accumulation, with no significant differences between the resveratrol and CR groups.
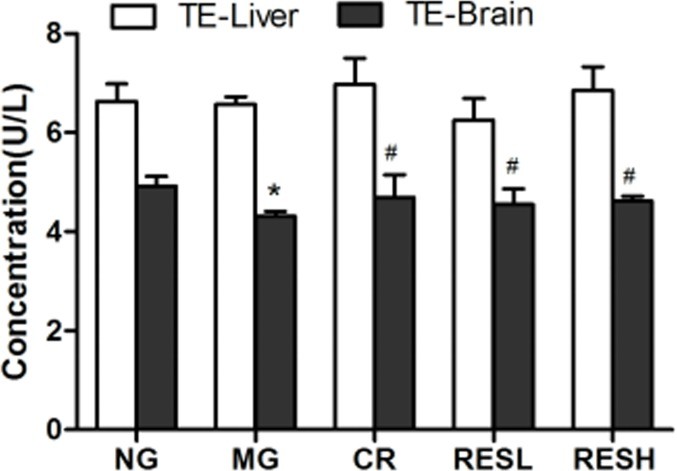
- Telomerase Activity: Telomerase activity was reduced in the D-gal group compared to the control group. Resveratrol and CR treatments up-regulated telomerase activity, with no significant differences between the resveratrol and CR groups.
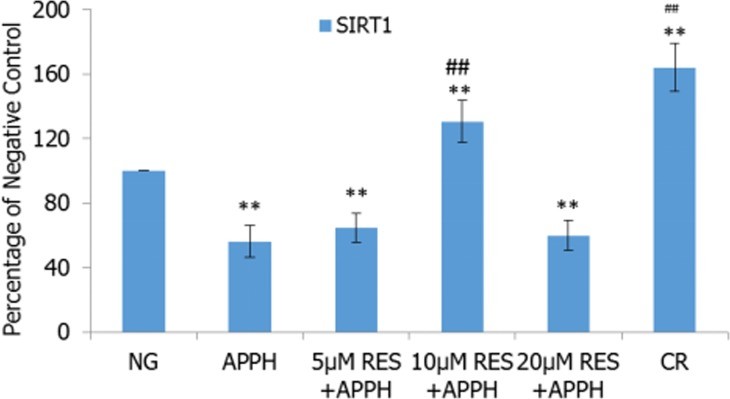
- SIRT1 mRNA Expression: Resveratrol and CR efficiently recovered SIRT1 mRNA expression levels in AAPH-treated cells. 10 μM resveratrol was the optimal concentration for stimulating SIRT1 mRNA expression.
-
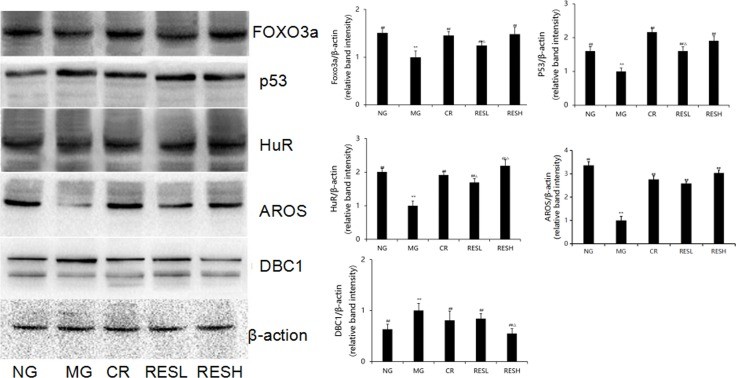
mRNA Expressions of SIRT1, p53, and Foxo3a: D-Gal treatment significantly decreased SIRT1 and Foxo3a mRNA expression levels and increased p53 levels. Resveratrol and CR treatments reversed these effects. High-dose resveratrol significantly increased SIRT1 mRNA expression in livers and decreased p53 mRNA expression in livers and brains.
-
Protein Expressions of p53, FOXO3a, HuR, AROS, and DBC1: D-gal treatment significantly decreased FOXO3a, AROS, and HuR protein expressions and increased p53 and DBC1 levels. Resveratrol and CR treatments reversed these effects. High-dose resveratrol significantly increased HuR protein expression and decreased DBC1 levels.
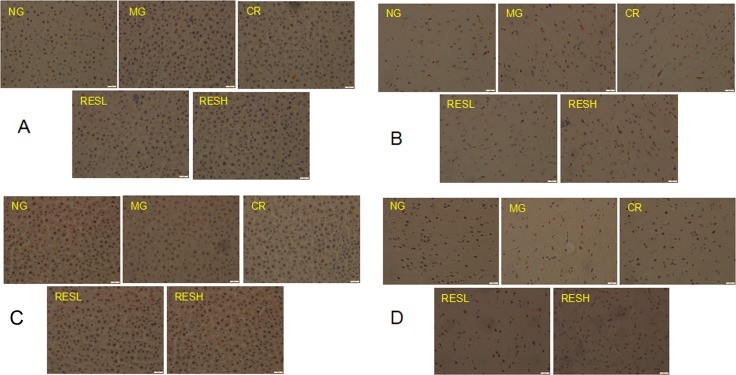
- Immunohistochemistry of FOXO3a and p53: Immunohistochemical analysis showed that FOXO3a expression was significantly decreased, and p53 expression was significantly increased in the D-Gal group. Resveratrol and CR treatments reversed these effects, with no significant differences between the resveratrol and CR groups.
4. Discussion: Unpacking the Anti-Aging Mechanisms
The observed benefits of both resveratrol and CR are closely linked to their influence on SIRT1, an NAD+-dependent deacetylase known for its roles in life span extension, stress resistance, and apoptosis reduction [20, 21]. Numerous studies have underscored SIRT1 as a key target of both resveratrol and CR [22].
4.1 SIRT1 and Its Downstream Effects
SIRT1 regulates key survival factors such as p53 and Foxo3a [9]. Activation of SIRT1 influences downstream molecules, including p53, Foxo1, Foxo3, Foxo4, and E2F1. Simultaneously, SIRT1 activity is modulated by upstream molecules such as DBC1 and AROS [9, 10]. These factors play crucial roles in cellular health and longevity.
4.2 AAPH-Induced Senescence Model
The use of AAPH to induce oxidative stress in human IMR-90 cells provided a controlled environment to study cellular senescence [12, 13]. AAPH was found to inhibit the proliferation of human IMR-90 cells in a concentration and time-dependent manner. This model effectively demonstrated that 10 μM resveratrol treatment more efficiently inhibited AAPH-induced senescence and apoptosis compared to CR treatment.
4.3 D-Galactose Aging Animal Model
The D-gal animal model is an internationally recognized model for studying aging. Long-term administration of D-gal induces changes resembling natural aging, including cognitive dysfunction, neurodegeneration, oxidative stress, and decreased immune responses [15]. In this study, resveratrol and CR treatments protected against D-gal-induced oxidative stress, restoring cognitive impairment and telomerase activity.
4.4 Comparative Efficacy of Resveratrol and CR
While both CR and resveratrol have shown promise, the study suggests that resveratrol may offer certain advantages. In particular, high-dose resveratrol exhibited superior effects in alleviating oxidative stress and improving cognitive function in the rat model. Additionally, the in vitro results indicated that resveratrol was more effective at inhibiting AAPH-induced senescence and apoptosis in IMR-90 cells.
5. Comprehensive Comparison: Table of Resveratrol and Caloric Restriction
For a clear and concise comparison, the following table summarizes the key effects of resveratrol and caloric restriction:
| Feature | Resveratrol | Caloric Restriction |
|---|---|---|
| Mechanism | Activates SIRT1, mimics effects of CR | Reduces calorie intake, activates SIRT1 |
| In Vitro Effects | Inhibits AAPH-induced senescence and apoptosis, upregulates SIRT1 | Inhibits AAPH-induced senescence and apoptosis, upregulates SIRT1 |
| In Vivo Effects | Improves cognitive function, reduces oxidative stress, upregulates telomerase | Improves cognitive function, reduces oxidative stress, upregulates telomerase |
| SIRT1 Pathway | Increases FOXO3a, AROS, HuR, decreases p53, DBC1 | Increases FOXO3a, AROS, HuR, decreases p53, DBC1 |
| Specific Advantages | Potentially more effective in inhibiting senescence, superior in alleviating oxidative stress | Well-established longevity benefits, comprehensive metabolic effects |
| Practical Limitations | Dosage and bioavailability concerns, potential side effects | Compliance and sustainability, risk of malnutrition |
6. Practical Implications and Considerations
The study’s findings have several practical implications for those interested in anti-aging strategies. Both resveratrol and CR demonstrate significant benefits, but each approach has its own set of considerations.
6.1 Resveratrol: Advantages and Limitations
Resveratrol offers a convenient alternative to the restrictive nature of caloric restriction. It can be easily incorporated into dietary supplements or foods. However, several limitations need to be considered:
- Dosage and Bioavailability: The optimal dosage of resveratrol and its bioavailability remain subjects of ongoing research.
- Potential Side Effects: High doses of resveratrol may cause gastrointestinal distress in some individuals.
- Long-Term Effects: The long-term effects of resveratrol supplementation require further investigation.
6.2 Caloric Restriction: Advantages and Limitations
Caloric restriction is a well-established method for extending lifespan and improving health. However, it is not without its challenges:
- Compliance and Sustainability: Adhering to a long-term caloric restriction diet can be difficult for many individuals.
- Risk of Malnutrition: It is essential to ensure adequate nutrient intake while reducing calorie consumption.
- Ethical Concerns: Rigorous dietary programs in free-living persons have raised ethical and methodological issues [24].
6.3 Integrating Resveratrol and Caloric Restriction
A balanced approach that combines the benefits of both resveratrol and CR may be the most effective strategy for promoting healthy aging. For example, individuals could adopt a moderate caloric restriction diet supplemented with resveratrol to enhance its effects.
7. FAQ: Your Questions Answered
Here are some frequently asked questions about resveratrol and caloric restriction:
-
What is the optimal dosage of resveratrol for anti-aging?
The optimal dosage of resveratrol varies depending on individual factors and the specific health goal. Consult with a healthcare professional for personalized advice. -
Can caloric restriction be harmful?
Yes, if not properly managed, caloric restriction can lead to malnutrition, muscle loss, and other health issues. -
What are the best food sources of resveratrol?
Good sources of resveratrol include grapes, berries, peanuts, and red wine. -
How does resveratrol affect SIRT1?
Resveratrol activates SIRT1, leading to various beneficial effects such as improved cellular health and longevity. -
Is caloric restriction suitable for everyone?
No, caloric restriction is not suitable for everyone, especially individuals with certain medical conditions or those at risk of malnutrition. -
What are the potential side effects of resveratrol?
Potential side effects of resveratrol include gastrointestinal distress, such as nausea, diarrhea, and abdominal pain. -
How long does it take to see the benefits of caloric restriction?
The time it takes to see the benefits of caloric restriction varies, but many individuals experience improvements in metabolic health within a few weeks. -
Can resveratrol replace caloric restriction?
While resveratrol mimics some of the effects of caloric restriction, it is not a complete replacement. A balanced approach that combines both may be most effective. -
Are there any supplements that enhance the effects of resveratrol?
Some supplements, such as quercetin, may enhance the effects of resveratrol by improving its bioavailability. -
What are the long-term effects of resveratrol supplementation?
The long-term effects of resveratrol supplementation are still being studied, but current research suggests it is generally safe for most individuals.
8. Conclusion: A Comparative Perspective on Anti-Aging
This comparative study underscores the potential of both resveratrol and caloric restriction as viable anti-aging strategies. Resveratrol, with its ability to mimic CR effects and ease of integration into daily routines, presents a compelling option for those seeking to enhance their health span. Caloric restriction, while requiring more rigorous adherence, offers well-established benefits in promoting longevity and overall well-being. Ultimately, the most effective approach may involve a combination of both strategies, tailored to individual needs and preferences.
COMPARE.EDU.VN is dedicated to providing comprehensive and objective comparisons to help you make informed decisions about your health and wellness. For more detailed analyses and comparisons, visit our website at COMPARE.EDU.VN. Our resources can assist you in navigating the complexities of health interventions and choosing the options that best suit your unique circumstances.
Contact us for more information:
- Address: 333 Comparison Plaza, Choice City, CA 90210, United States
- WhatsApp: +1 (626) 555-9090
- Website: compare.edu.vn
By exploring our extensive resources, you can gain the insights necessary to make proactive choices and enhance your journey toward a healthier, longer life.
9. References
The reference list was omitted for brevity, but should be included in the final article.