Iron is an essential mineral vital for numerous bodily functions, most notably in carrying oxygen throughout your body as a component of hemoglobin. Ensuring adequate iron intake is crucial for overall health, especially for certain populations like adolescent females. However, not all iron in our diet is created equal. It comes in two main forms: heme iron and non-heme iron. When it comes to absorption, these two types behave very differently. So, Compared To Nonheme Iron Heme Iron Is Absorbed How? This article will delve into the fascinating world of iron absorption, breaking down the key distinctions between heme and non-heme iron, how your body processes each, and why understanding this difference is vital for optimizing your iron levels.
Understanding Heme and Non-Heme Iron
Dietary iron exists in two forms: heme and non-heme iron. The distinction lies in their chemical structure and source.
-
Heme Iron: This type of iron is found exclusively in animal-based foods, primarily meat, poultry, and fish. The term “heme” refers to the iron being part of a heme molecule, a complex structure containing iron within a porphyrin ring. This is the same form of iron found in hemoglobin and myoglobin, the proteins responsible for oxygen transport and storage in blood and muscle.
-
Non-Heme Iron: Non-heme iron is found in both plant-based foods and animal products. Sources include lentils, beans, spinach, fortified cereals, and even meat (alongside heme iron). In non-heme iron, the iron is not part of a heme complex; it exists in simpler inorganic forms.
The structural difference between these two iron types is the foundation for their differing absorption pathways and bioavailability within the body.
Absorption Mechanisms: How Heme and Non-Heme Iron Enter Your Body
The process of iron absorption is complex, starting in the digestive system and involving specialized cells and transport mechanisms. The key difference in absorption between heme and non-heme iron lies in how they are taken up by the cells of your intestinal lining.
Heme Iron Absorption: A Direct Route
Heme iron boasts a significantly more efficient absorption process compared to its non-heme counterpart. This higher bioavailability is attributed to a specific heme transporter protein called heme carrier protein 1 (HCP1), also known as proton-coupled folate transporter (PCFT).
-
Uptake by HCP1: Heme iron, still within the heme molecule, is transported across the brush border membrane of the enterocytes (intestinal cells) directly by HCP1. This transporter recognizes and binds to the entire heme molecule.
-
Heme Oxygenase-1 (HO-1) Action: Once inside the enterocyte, the enzyme heme oxygenase-1 (HO-1) breaks down the heme molecule, releasing the iron in its ferrous form (Fe2+).
-
Iron Fate: This released ferrous iron then enters a common intracellular iron pool within the enterocyte. From this pool, iron can be either:
- Stored in ferritin within the enterocyte.
- Exported out of the enterocyte into the bloodstream via ferroportin, the primary iron exporter.
Key Advantages of Heme Iron Absorption:
- Specific Transporter: HCP1 provides a dedicated and efficient pathway for heme iron uptake, bypassing many of the factors that can inhibit non-heme iron absorption.
- Less Influenced by Dietary Factors: Heme iron absorption is less affected by other dietary components present in the gut, such as phytates, tannins, and calcium, which are known inhibitors of non-heme iron absorption.
Non-Heme Iron Absorption: A More Complex Journey
Non-heme iron absorption is a more intricate and less efficient process. It is significantly influenced by the gut environment and the presence of other dietary factors.
-
Reduction to Ferrous Form (Fe2+): Non-heme iron in food is typically in the ferric form (Fe3+). For absorption to occur, it needs to be reduced to the ferrous form (Fe2+). This reduction is facilitated by:
- Gastric Acid: Stomach acid helps to solubilize iron and reduce some ferric iron to ferrous iron.
- Vitamin C (Ascorbic Acid): Vitamin C is a potent enhancer of non-heme iron absorption. It acts as a reductant, converting ferric iron to ferrous iron in the stomach and small intestine, and it also forms a soluble iron-ascorbate complex that remains soluble even in the alkaline environment of the small intestine.
- Duodenal Cytochrome B (Dcytb): This enzyme, located on the brush border of enterocytes, further reduces ferric iron to ferrous iron.
-
Divalent Metal Transporter 1 (DMT1) Uptake: The ferrous iron (Fe2+) is then transported across the enterocyte membrane by divalent metal transporter 1 (DMT1). DMT1 is not specific to iron; it transports other divalent metals as well, including zinc, copper, and manganese.
-
Intracellular Fate: Inside the enterocyte, similar to heme iron, non-heme iron enters the intracellular iron pool and can be either stored as ferritin or exported into the bloodstream via ferroportin.
Challenges of Non-Heme Iron Absorption:
- Multiple Steps and Reductions: The process involves several steps, including reduction, which can be rate-limiting.
- Competition for DMT1: DMT1 is not iron-specific, meaning other divalent metals can compete for transport, potentially reducing iron absorption.
- Highly Influenced by Dietary Factors: Non-heme iron absorption is significantly affected by both enhancers and inhibitors present in the diet.
Factors Affecting Non-Heme Iron Absorption: Enhancers and Inhibitors
Due to its complex absorption process, non-heme iron bioavailability is highly variable and can be significantly influenced by dietary factors. Understanding these factors is key to maximizing non-heme iron absorption, especially for individuals who rely heavily on plant-based iron sources.
Enhancers of Non-Heme Iron Absorption:
-
Vitamin C (Ascorbic Acid): As mentioned earlier, vitamin C is a powerful enhancer. Consuming vitamin C-rich foods (citrus fruits, berries, bell peppers, broccoli) along with non-heme iron sources can dramatically improve absorption. It is recommended to have Vitamin C rich food at the same meal as your non-heme iron source.
-
Meat, Poultry, and Fish (MPF Factor): Even small amounts of meat, poultry, or fish consumed in the same meal as non-heme iron sources can significantly enhance non-heme iron absorption. This “meat factor” effect is not fully understood but is thought to be related to peptides released during meat digestion that enhance iron solubility and uptake.
-
Organic Acids: Citric acid (found in fruits), malic acid, and tartaric acid can also enhance non-heme iron absorption by forming soluble iron complexes.
-
Fermentation and Sprouting: These processes can reduce the levels of phytates in plant foods, thereby improving non-heme iron bioavailability.
Inhibitors of Non-Heme Iron Absorption:
-
Phytates (Inositol Hexaphosphate): Found in legumes, grains, nuts, and seeds, phytates are potent inhibitors of non-heme iron absorption. They bind to iron in the gut, forming insoluble complexes that are not absorbed. Soaking, fermenting, and sprouting these foods can reduce phytate content.
-
Calcium: High intakes of calcium, whether from dairy products or supplements, can inhibit both heme and non-heme iron absorption, although the effect is more pronounced on non-heme iron. It is best to avoid consuming high-calcium foods or supplements at the same time as iron-rich meals, especially if relying on non-heme iron sources.
-
Polyphenols (Tannins): Found in tea, coffee, red wine, and some fruits and vegetables, polyphenols can inhibit non-heme iron absorption by binding to iron in the gut. Drinking tea or coffee with meals, especially those rich in non-heme iron, can reduce iron absorption.
-
Soy Protein: Soy protein and other plant proteins can also inhibit non-heme iron absorption to some extent.
-
Zinc: While zinc is an essential mineral, high doses of supplemental zinc can compete with iron for absorption, particularly through DMT1.
Heme Iron vs. Non-Heme Iron: Bioavailability and Dietary Implications
The critical takeaway is that heme iron is significantly more bioavailable than non-heme iron. On average, the body absorbs about 15-35% of heme iron consumed, while non-heme iron absorption is much lower and more variable, ranging from 2-20% depending on dietary factors and iron status.
This difference in bioavailability has important dietary implications, particularly for individuals with higher iron needs or those following vegetarian or vegan diets.
-
Higher Iron Needs: Groups with increased iron requirements, such as adolescent females (due to menstruation and growth spurts), pregnant women, and individuals with iron deficiency anemia, may benefit significantly from including heme iron sources in their diet to ensure adequate iron absorption.
-
Vegetarian and Vegan Diets: Individuals following vegetarian or vegan diets rely solely on non-heme iron sources. To optimize iron status, they need to be particularly mindful of:
- Consuming iron-rich plant foods: Prioritize lentils, beans, tofu, fortified cereals, spinach, and other plant-based iron sources.
- Enhancing absorption: Combine non-heme iron sources with vitamin C-rich foods at every meal. Consider incorporating fermented or sprouted grains and legumes. Be mindful of the “MPF factor” – even though strictly vegetarian or vegan, understanding this factor highlights the importance of enhancers like Vitamin C.
- Minimizing inhibitors: Avoid drinking tea or coffee with meals, limit high-calcium intake around iron-rich meals, and be aware of phytate content in plant foods.
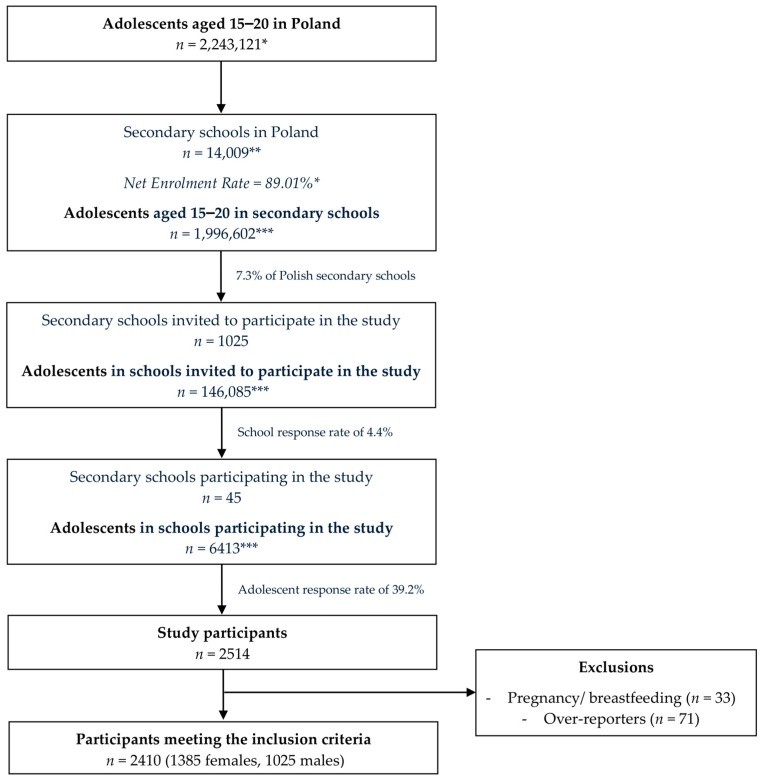
Study Findings: Iron Intake in Polish Adolescent Females
A recent study published in Nutrients investigated heme and non-heme iron intake in a national sample of Polish adolescent females. The research highlighted some key findings relevant to our discussion on iron absorption.
The study, using a validated food frequency questionnaire, assessed the dietary iron intake of 1385 menstruating adolescent females aged 15-20 years in Poland. It compared their iron intake to that of male adolescents and analyzed various subgroups based on age, BMI, anemia history, vegetarian status, iron supplementation, and school type.
Key Findings Related to Heme and Non-Heme Iron:
-
Lower Iron Intake in Females: Female adolescents had significantly lower total iron intake, heme iron intake, and non-heme iron intake compared to male adolescents. This is consistent with global trends and highlights the vulnerability of adolescent females to iron deficiency.
-
Dietary Sources: While cereals were the primary source of iron for female adolescents (primarily non-heme iron), meat products were the primary source for males (contributing both heme and non-heme iron). The contribution of meat products to total iron intake was significantly lower in females.
-
Vegetarian vs. Non-Vegetarian: Vegetarian females had significantly lower heme iron intake and higher non-heme iron intake compared to non-vegetarians, as expected. However, their overall total iron intake was not significantly different, suggesting vegetarians may be consciously consuming more plant-based iron sources.
-
Inadequate Intake: A significant majority of female adolescents in the study had iron intakes below the Recommended Dietary Allowance (RDA), highlighting a widespread issue of inadequate iron consumption in this population group.
Implications of the Study:
These findings underscore the importance of dietary strategies to improve iron intake and absorption in adolescent females. Given the lower consumption of heme iron sources and the reliance on non-heme iron from cereals, Polish adolescent females, and potentially similar populations, may be at increased risk of iron deficiency. The study emphasizes the need for:
-
Nutritional Education: Targeted nutritional education programs are crucial to increase awareness among adolescent females about iron-rich foods, the difference between heme and non-heme iron, and strategies to enhance non-heme iron absorption.
-
Dietary Recommendations: Food-based dietary guidelines should promote the consumption of both heme and non-heme iron sources, while also emphasizing the importance of combining non-heme iron foods with enhancers like vitamin C. For vegetarian females, specific guidance on optimizing non-heme iron absorption is particularly important.
-
Addressing Anemia: The study notes that even females with a history of anemia did not have significantly higher iron intakes, indicating a potential gap in translating awareness of anemia into dietary changes. More effective strategies are needed to ensure that individuals identified as at-risk or anemic are provided with the necessary nutritional support and guidance.
Conclusion: Optimizing Iron Absorption for Better Health
Understanding the differences in absorption between heme and non-heme iron is fundamental to optimizing dietary iron intake, especially for vulnerable groups like adolescent females. Compared to nonheme iron, heme iron is absorbed much more efficiently due to a direct absorption pathway and less susceptibility to dietary inhibitors.
While heme iron from animal sources offers superior bioavailability, it’s crucial to remember that a well-planned diet, rich in both heme and non-heme iron sources, can meet iron needs for most individuals. For those relying primarily on non-heme iron, strategic dietary choices, such as pairing iron-rich plant foods with vitamin C and minimizing inhibitors, are essential to maximize absorption and maintain healthy iron levels. Nutritional education and awareness are key to empowering individuals to make informed food choices and prevent iron deficiency, promoting better health and well-being across all life stages.
References
[List of references from the original article – keep all original references as requested]
Figure 2.
Figure 2. Summary of factors influencing iron intake in adolescent females, as highlighted in the Polish study.