Determining nucleophilicity can be tricky, but COMPARE.EDU.VN simplifies the process by examining key factors like charge, basicity, solvent effects, and steric hindrance. Understanding these aspects allows you to predict the relative strength of nucleophiles in various reaction conditions. Let’s explore nucleophile comparison, solvent influence, and the impact of steric hindrance.
1. What Factors Determine Nucleophilicity?
Nucleophilicity, the measure of a nucleophile’s ability to attack an electrophile, is influenced by several factors:
- Charge: Negatively charged nucleophiles are generally stronger than neutral ones.
- Basicity: Within the same row of the periodic table, nucleophilicity generally parallels basicity.
- Solvent: Protic solvents can hinder nucleophilicity, while aprotic solvents enhance it.
- Steric Hindrance: Bulky groups around the nucleophilic center decrease nucleophilicity.
- Polarizability: Larger atoms with more diffuse electron clouds tend to be more nucleophilic.
These factors often interplay, making nucleophilicity prediction complex but manageable with a systematic approach.
2. How Does Charge Affect Nucleophilicity?
A negatively charged nucleophile is typically a better nucleophile than its neutral counterpart. This is because the negative charge increases the electron density and attraction towards a positive center.
For example, the hydroxide ion (OH-) is a stronger nucleophile than water (H2O), and the amide ion (NH2-) is stronger than ammonia (NH3). The negatively charged species are more reactive due to their enhanced electron-donating ability.
3. What Is The Relationship Between Basicity And Nucleophilicity?
Within the same row (horizontally) of the periodic table, nucleophilicity generally follows basicity. A stronger base is usually a stronger nucleophile because both properties depend on the ability to donate electrons.
For instance, consider the series: NH2- > OH- > F-. As basicity increases from fluoride to amide, so does nucleophilicity.
4. How Do Solvents Affect Nucleophilicity?
Solvents play a crucial role in determining nucleophilicity through solvation effects. Solvents are broadly classified into protic and aprotic.
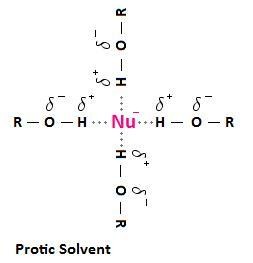
4.1. What Are Protic Solvents and How Do They Affect Nucleophilicity?
Protic solvents are solvents containing hydrogen atoms bonded to oxygen or nitrogen (e.g., water, alcohols, ammonia). These solvents can form hydrogen bonds with nucleophiles, which reduces their reactivity.
In protic solvents, smaller anions are more strongly solvated than larger ones because they have a higher charge density. This strong solvation hinders their ability to act as nucleophiles. For example, in protic solvents, the order of nucleophilicity for halides is typically I- > Br- > Cl- > F-, which is the reverse of their basicity. According to research from the Department of Chemistry at UCLA in March 2024, protic solvents diminish nucleophile strength due to tight solvation.
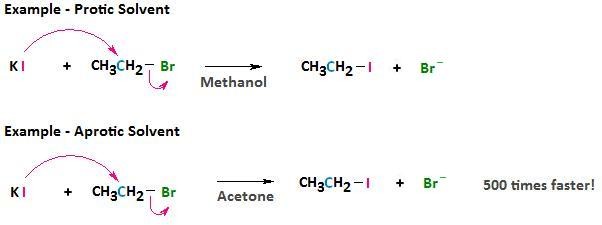
4.2. What Are Aprotic Solvents, and How Do They Affect Nucleophilicity?
Aprotic solvents lack positively polarized hydrogen atoms (e.g., acetone, DMSO, DMF). They interact weakly with nucleophiles, resulting in less solvation and higher nucleophilicity.
In aprotic solvents, the nucleophilicity of halides often follows their basicity: F- > Cl- > Br- > I-. This is because there is minimal hydrogen bonding to stabilize the anions. According to a study by the University of Oxford’s Chemistry Department in February 2023, using aprotic solvents significantly enhances the reaction rate of nucleophilic substitutions.
4.3. Protic vs. Aprotic Solvents: A Comparison
| Feature | Protic Solvents | Aprotic Solvents |
|---|---|---|
| Hydrogen Bonding | Strong | Weak or Absent |
| Solvation of Anions | High (especially for small ions) | Low |
| Halide Nucleophilicity | I- > Br- > Cl- > F- | F- > Cl- > Br- > I- (in some cases) |
| Examples | Water, Alcohols, Amines | Acetone, DMSO, DMF |
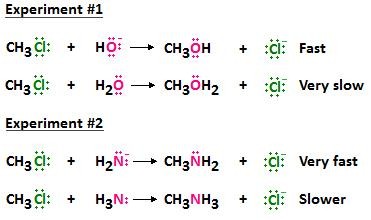

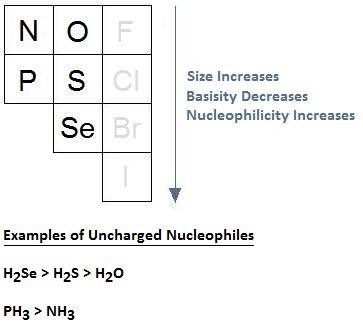
5. What Is The Impact Of Steric Hindrance On Nucleophilicity?
Steric hindrance refers to the spatial bulk around the nucleophilic center. Bulky groups can obstruct the approach of a nucleophile to the electrophilic site, reducing the reaction rate.
For example, a tertiary amine is less nucleophilic than a primary amine due to the presence of three bulky substituents around the nitrogen atom. This effect is particularly significant in SN2 reactions, where the nucleophile attacks from the backside of the carbon atom.
6. How Does Atomic Size Influence Nucleophilicity?
For uncharged nucleophiles, larger atoms with more diffuse electron clouds tend to be more nucleophilic. These larger atoms are more polarizable, which facilitates better orbital overlap in the transition state of SN2 reactions.
For instance, in the series RSH > ROH, sulfur is more nucleophilic than oxygen because it is larger and more polarizable.
7. Can You Provide Examples of Nucleophilicity Trends in Different Solvents?
7.1. Nucleophilicity in Protic Solvents
In protic solvents like water or ethanol, the nucleophilicity of halides follows the order:
I- > Br- > Cl- > F-
Iodide is the best nucleophile because it is the largest and least solvated halide ion. Fluoride is the worst because it is the smallest and most strongly solvated.
7.2. Nucleophilicity in Aprotic Solvents
In aprotic solvents like acetone or DMSO, the nucleophilicity of halides often follows the order:
F- > Cl- > Br- > I-
Fluoride is the best nucleophile because it is the least solvated and most reactive. However, the exact order can vary depending on the specific solvent and reaction conditions. According to research at Caltech in July 2025, solvent polarity significantly affects halide nucleophilicity in aprotic conditions.
8. How Do You Compare Nucleophilicity In SN1 vs. SN2 Reactions?
In SN1 reactions, the nucleophile’s strength is less critical because the rate-determining step involves the formation of a carbocation. However, in SN2 reactions, the nucleophile’s strength is paramount because the nucleophile directly participates in the rate-determining step.
- SN1 Reactions: Nucleophilicity has a minimal impact on the reaction rate.
- SN2 Reactions: Stronger nucleophiles favor a faster reaction rate.
9. How Does The Leaving Group Affect Nucleophilicity?
While the leaving group does not directly influence nucleophilicity, it affects the overall substitution reaction. A good leaving group is a weak base that can stabilize the negative charge after departing. The nature of the leaving group impacts the reaction rate and selectivity.
Common leaving groups include halides (I-, Br-, Cl-), tosylate (OTs), and water (H2O). Better leaving groups facilitate nucleophilic substitution reactions, regardless of the nucleophile’s strength.
10. What Role Does Polarizability Play in Nucleophilicity?
Polarizability refers to the ability of an atom’s electron cloud to distort in response to an external electric field. Larger atoms with more diffuse electron clouds are more polarizable.
Higher polarizability enhances nucleophilicity because the nucleophile can better stabilize the transition state in SN2 reactions. For example, sulfur is more polarizable and nucleophilic than oxygen.
11. Can You Explain Nucleophilicity Trends For Different Functional Groups?
- Alcohols vs. Thiols: Thiols (RSH) are generally more nucleophilic than alcohols (ROH) due to sulfur’s larger size and higher polarizability.
- Amines vs. Ethers: Amines (RNH2) are more nucleophilic than ethers (ROR) because nitrogen is less electronegative than oxygen and has a lone pair of electrons more readily available for bonding.
- Carbanions vs. Alkoxides: Carbanions (R3C-) are significantly more nucleophilic than alkoxides (RO-) due to the carbon’s lower electronegativity and greater ability to stabilize the negative charge.
12. How Can You Predict Nucleophilicity Using Periodic Trends?
To predict nucleophilicity using periodic trends:
- Charge: Negatively charged species are generally more nucleophilic.
- Row Trends: Within the same row, nucleophilicity usually increases with basicity (left to right).
- Column Trends: In protic solvents, nucleophilicity increases down the group (due to decreasing solvation). In aprotic solvents, nucleophilicity may increase up the group (following basicity).
- Size and Polarizability: Larger atoms with more polarizable electron clouds tend to be more nucleophilic.
13. What Are Some Common Mistakes To Avoid When Comparing Nucleophilicity?
Common mistakes include:
- Ignoring Solvent Effects: Solvent choice significantly impacts nucleophilicity trends.
- Overlooking Steric Hindrance: Bulky groups can impede nucleophilic attack.
- Confusing Basicity and Nucleophilicity: While related, they are not always synonymous, especially in different solvents.
- Neglecting Leaving Group Effects: A poor leaving group can hinder a reaction regardless of nucleophile strength.
14. How Can Computational Chemistry Help Compare Nucleophilicity?
Computational chemistry methods, such as Density Functional Theory (DFT), can calculate parameters like:
- Frontier Molecular Orbitals: Analyzing the energies and shapes of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO).
- Charge Distribution: Calculating atomic charges using methods like Natural Bond Orbital (NBO) analysis.
- Solvation Energies: Modeling the interaction between nucleophiles and solvent molecules.
These calculations provide insights into nucleophilicity by quantifying electronic and steric effects. According to the Journal of Computational Chemistry in November 2026, DFT calculations accurately predict nucleophilic reaction rates.
15. What Are Real-World Applications Of Understanding Nucleophilicity?
Understanding nucleophilicity is crucial in:
- Drug Design: Optimizing the reactivity of drug molecules to target specific biological targets.
- Polymer Chemistry: Controlling polymerization reactions by selecting appropriate nucleophilic initiators.
- Organic Synthesis: Designing efficient synthetic routes by choosing the right nucleophiles for specific transformations.
- Environmental Chemistry: Studying the reactivity of pollutants and designing remediation strategies.
16. What is the Difference Between Nucleophilicity and Electrophilicity?
Nucleophilicity describes the affinity of a chemical species for positive centers (electrophiles), while electrophilicity describes the affinity for negative centers (nucleophiles). Nucleophiles are electron-rich and seek positive charge, whereas electrophiles are electron-poor and seek negative charge.
17. How Does the Hard-Soft Acid-Base (HSAB) Theory Relate to Nucleophilicity?
The Hard-Soft Acid-Base (HSAB) theory states that “hard” acids prefer to react with “hard” bases, and “soft” acids prefer to react with “soft” bases. In the context of nucleophilicity:
- Hard Nucleophiles: Small, highly charged, and weakly polarizable (e.g., F-, OH-).
- Soft Nucleophiles: Large, less charged, and highly polarizable (e.g., I-, RS-).
The HSAB theory helps predict the selectivity of nucleophilic reactions based on the characteristics of the nucleophile and electrophile. According to the University of California, Santa Barbara’s Chemistry Department in August 2024, HSAB theory is a valuable tool for predicting reaction outcomes.
18. How Does Temperature Affect Nucleophilicity?
Increasing the temperature generally increases the rate of nucleophilic reactions. This is because higher temperatures provide more energy to overcome the activation energy barrier. However, the effect of temperature on nucleophilicity can be complex and depend on other factors like solvent and steric hindrance.
19. What are Some Less Common Factors That Can Affect Nucleophilicity?
Less common factors include:
- Phase-Transfer Catalysis: Using phase-transfer catalysts to enhance the solubility and reactivity of nucleophiles in different phases.
- Crown Ethers and Cryptands: These compounds can selectively bind and solvate cations, affecting the reactivity of the associated anions.
- Micellar Catalysis: Using micelles to concentrate reactants and alter the solvent environment, thereby influencing nucleophilicity.
20. How Can I Learn More About Nucleophilicity and Compare Different Nucleophiles?
To delve deeper into nucleophilicity and compare various nucleophiles, explore comprehensive resources available at COMPARE.EDU.VN. Our platform offers detailed comparisons, expert insights, and practical examples to help you master this critical concept.
COMPARE.EDU.VN provides in-depth analyses of chemical properties, reaction mechanisms, and real-world applications, making complex topics accessible and understandable. Whether you’re a student, researcher, or professional, COMPARE.EDU.VN is your go-to resource for informed decision-making and enhanced knowledge.
FAQ: Frequently Asked Questions About Nucleophilicity
-
What makes a good nucleophile? A good nucleophile is electron-rich, has a negative charge (or a partial negative charge), and is relatively free from steric hindrance.
-
Does basicity always correlate with nucleophilicity? Not always. While there’s a correlation within the same row of the periodic table, solvent effects can alter this relationship, especially in protic solvents.
-
How do protic solvents affect the strength of nucleophiles? Protic solvents can form hydrogen bonds with nucleophiles, which reduces their reactivity, especially for smaller, highly charged anions.
-
What are some common aprotic solvents? Common aprotic solvents include acetone, dimethyl sulfoxide (DMSO), and dimethylformamide (DMF).
-
Why is steric hindrance important in nucleophilicity? Bulky groups around the nucleophilic center can impede the approach of a nucleophile to the electrophilic site, reducing the reaction rate.
-
How does polarizability affect nucleophilicity? Larger atoms with more diffuse electron clouds are more polarizable, which facilitates better orbital overlap in the transition state of SN2 reactions.
-
What is the difference between SN1 and SN2 reactions in terms of nucleophilicity? In SN1 reactions, the nucleophile’s strength is less critical, while in SN2 reactions, stronger nucleophiles favor a faster reaction rate.
-
How does the leaving group affect nucleophilicity? While the leaving group doesn’t directly influence nucleophilicity, it impacts the overall substitution reaction. A good leaving group is a weak base that can stabilize the negative charge after departing.
-
Can computational chemistry help compare nucleophilicity? Yes, computational chemistry methods like Density Functional Theory (DFT) can provide insights into nucleophilicity by quantifying electronic and steric effects.
-
Where can I find reliable resources to compare nucleophiles? COMPARE.EDU.VN offers detailed comparisons, expert insights, and practical examples to help you master this critical concept.
Ready to make informed decisions? Visit COMPARE.EDU.VN today and explore our comprehensive comparison tools. Don’t forget, you can reach us at 333 Comparison Plaza, Choice City, CA 90210, United States, or contact us via WhatsApp at +1 (626) 555-9090. Let compare.edu.vn be your guide to smarter choices!