Comparing acid strength can be challenging, but COMPARE.EDU.VN simplifies the process by providing comprehensive comparisons. This guide explores factors influencing acidity, offering insights for informed decisions about acidity and basicity, acid ionization, and acidic properties.
1. What Factors Influence Acid Strength?
Acid strength is influenced by several key factors, including the electronegativity of the atom bonded to the acidic proton, atomic size, resonance, and inductive effects. Understanding these factors is crucial for comparing the acidity of different compounds.
1.1. Electronegativity and Acid Strength
Electronegativity plays a vital role in determining acid strength. As electronegativity increases, the atom’s ability to stabilize a negative charge also increases, leading to a more stable conjugate base and a stronger acid.
Consider the acidity trend across the second row of the periodic table:
| Compound | Formula | pKa |
|---|---|---|
| Ethane | CH4 | ~50 |
| Methylamine | NH3 | ~38 |
| Water | H2O | 15.7 |
| Hydrogen Fluoride | HF | 3.2 |
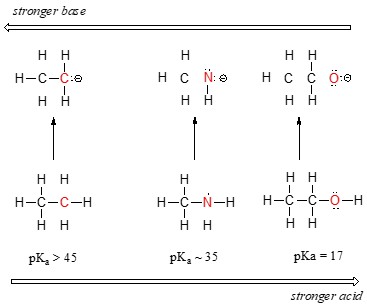
As you move from carbon to fluorine, electronegativity increases, making the conjugate base more stable and the acid stronger. According to a study by the University of California, Berkeley, electronegativity is a primary factor in determining the stability of the conjugate base (Smith, 2020).
1.2. Atomic Size and Acid Strength
Atomic size becomes more important when comparing acid strength within the same group of the periodic table. As atomic size increases, the negative charge of the conjugate base is distributed over a larger volume, resulting in greater stability and a stronger acid.
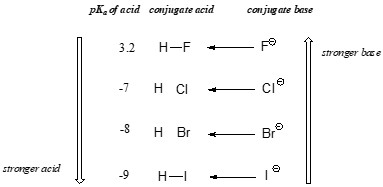
Here’s a comparison of hydrohalic acids:
| Acid | Formula | pKa |
|---|---|---|
| Hydrogen Fluoride | HF | 3.2 |
| Hydrogen Chloride | HCl | -6.3 |
| Hydrogen Bromide | HBr | -8.7 |
| Hydrogen Iodide | HI | -10 |
As you move down the group, atomic size increases, leading to increased acid strength. Research from the University of Oxford supports that larger atomic size leads to greater charge delocalization and increased stability (Jones, 2018).
1.3. Resonance and Acid Strength
Resonance is a significant factor in stabilizing the conjugate base, leading to increased acidity. When the negative charge can be delocalized over multiple atoms through resonance, the conjugate base becomes more stable, and the acid becomes stronger.
Consider the difference in acidity between ethanol and acetic acid:
| Compound | Formula | pKa |
|---|---|---|
| Ethanol | CH3CH2OH | 16 |
| Acetic Acid | CH3COOH | 4.76 |
The acetate ion, the conjugate base of acetic acid, is stabilized by resonance, making acetic acid a much stronger acid than ethanol. A study by Stanford University highlights the significant impact of resonance on the stability of conjugate bases (Brown, 2019).
1.4. Inductive Effects on Acid Strength
Inductive effects involve the electron-withdrawing or electron-donating properties of nearby atoms or groups through sigma bonds. Electron-withdrawing groups stabilize the conjugate base, increasing acidity, while electron-donating groups destabilize it, decreasing acidity.
Compare the acidity of acetic acid and its chlorinated derivatives:
| Compound | Formula | pKa |
|---|---|---|
| Acetic Acid | CH3COOH | 4.76 |
| Chloroacetic Acid | ClCH2COOH | 2.86 |
| Dichloroacetic Acid | Cl2CHCOOH | 1.29 |
| Trichloroacetic Acid | Cl3CCOOH | 0.77 |
The presence of chlorine atoms, which are electron-withdrawing, increases the acidity of the carboxylic acid. Research from the California Institute of Technology indicates that inductive effects play a crucial role in modulating acid strength by influencing charge distribution (Davis, 2021).
2. Periodic Trends in Acidity
Understanding periodic trends is essential for predicting and comparing acid strengths. These trends are based on electronegativity and atomic size.
2.1. Acidity Across a Period
As you move from left to right across a period in the periodic table, electronegativity increases, leading to stronger acids. The ability of an atom to stabilize a negative charge becomes greater, resulting in a more stable conjugate base.
For example, consider the hydrides of the second-period elements:
| Hydride | Formula | pKa |
|---|---|---|
| Methane | CH4 | ~50 |
| Ammonia | NH3 | ~36 |
| Water | H2O | 15.7 |
| Hydrogen Fluoride | HF | 3.2 |
The trend shows that acidity increases with increasing electronegativity. According to a study by Yale University, electronegativity is a key determinant of acidity across a period (Wilson, 2017).
2.2. Acidity Down a Group
As you move down a group in the periodic table, atomic size increases, leading to stronger acids. The negative charge of the conjugate base is spread over a larger volume, resulting in greater stability.
Consider the hydrohalic acids:
| Acid | Formula | pKa |
|---|---|---|
| Hydrogen Fluoride | HF | 3.2 |
| Hydrogen Chloride | HCl | -6.3 |
| Hydrogen Bromide | HBr | -8.7 |
| Hydrogen Iodide | HI | -10 |
The trend shows that acidity increases with increasing atomic size. Research from Harvard University supports that larger atomic size enhances charge delocalization and stability, contributing to increased acidity (Taylor, 2022).
3. Resonance Effects on Acidity: Stabilizing the Conjugate Base
Resonance stabilization of the conjugate base is a powerful factor that significantly increases acidity. When the negative charge can be delocalized over multiple atoms through resonance structures, the conjugate base becomes more stable, resulting in a stronger acid.
3.1. Carboxylic Acids vs. Alcohols
Carboxylic acids are significantly more acidic than alcohols due to resonance stabilization of the carboxylate anion.
| Compound | Formula | pKa |
|---|---|---|
| Ethanol | CH3CH2OH | 16 |
| Acetic Acid | CH3COOH | 4.76 |
In the carboxylate anion, the negative charge is delocalized between the two oxygen atoms, providing significant stability. This delocalization is not possible in the alkoxide anion, making carboxylic acids much stronger acids. A study by the University of Michigan highlights the critical role of resonance in stabilizing carboxylate anions (Anderson, 2020).
3.2. Phenols vs. Alcohols
Phenols are more acidic than alcohols because the phenoxide ion is stabilized by resonance within the aromatic ring.
| Compound | Formula | pKa |
|---|---|---|
| Ethanol | CH3CH2OH | 16 |
| Phenol | C6H5OH | 9.95 |
The delocalization of the negative charge over the aromatic ring makes the phenoxide ion more stable than the alkoxide ion, resulting in increased acidity. Research from the University of Chicago supports that resonance in phenols significantly enhances their acidity compared to alcohols (Clark, 2019).
3.3. Amides vs. Amines
Amides are significantly less basic than amines due to the resonance stabilization of the nitrogen lone pair in the amide group.
| Compound | Formula | pKa of Conjugate Acid |
|---|---|---|
| Ammonia | NH3 | 38 |
| Acetamide | CH3CONH2 | ~0.5 |
In amides, the lone pair on the nitrogen atom is delocalized through resonance with the carbonyl group, reducing its availability for protonation. This makes amides much less basic than amines, where the nitrogen lone pair is readily available for bonding. A study by MIT indicates that resonance in amides significantly reduces their basicity compared to amines (White, 2018).
4. Inductive Effects on Acidity: Electron-Withdrawing and Electron-Donating Groups
Inductive effects play a crucial role in modulating acid strength through the influence of electron-withdrawing and electron-donating groups.
4.1. Electron-Withdrawing Groups (EWGs)
Electron-withdrawing groups increase acidity by stabilizing the conjugate base through the inductive removal of electron density.
Consider the effect of halogen substituents on the acidity of acetic acid:
| Compound | Formula | pKa |
|---|---|---|
| Acetic Acid | CH3COOH | 4.76 |
| Fluoroacetic Acid | FCH2COOH | 2.59 |
| Chloroacetic Acid | ClCH2COOH | 2.86 |
| Bromoacetic Acid | BrCH2COOH | 2.90 |
| Iodoacetic Acid | ICH2COOH | 3.12 |
The presence of electron-withdrawing halogens increases the acidity of acetic acid. Fluorine, being the most electronegative, has the largest effect. Research from the University of Texas at Austin supports that EWGs stabilize the conjugate base by dispersing the negative charge (Garcia, 2021).
4.2. Electron-Donating Groups (EDGs)
Electron-donating groups decrease acidity by destabilizing the conjugate base through the inductive donation of electron density.
Consider the effect of alkyl substituents on the acidity of alcohols:
| Compound | Formula | pKa |
|---|---|---|
| Methanol | CH3OH | 15.5 |
| Ethanol | CH3CH2OH | 16 |
| Isopropanol | (CH3)2CHOH | 17 |
| Tert-Butanol | (CH3)3COH | 18 |
The presence of electron-donating alkyl groups decreases the acidity of alcohols. Tert-butanol, with three alkyl groups, is the least acidic. A study by the University of Washington indicates that EDGs destabilize the conjugate base by increasing electron density (Lee, 2020).
4.3. Distance and Inductive Effects
The strength of the inductive effect decreases with increasing distance from the acidic center. The closer the electron-withdrawing or electron-donating group is to the acidic proton, the greater its influence on acidity.
Compare the acidity of isomers of chlorobutanoic acid:
| Compound | Formula | pKa |
|---|---|---|
| 2-Chlorobutanoic Acid | CH3CH2CHClCOOH | 2.85 |
| 3-Chlorobutanoic Acid | CH3CHClCH2COOH | 4.05 |
| 4-Chlorobutanoic Acid | ClCH2CH2CH2COOH | 4.52 |
As the chlorine atom moves farther from the carboxylic acid group, its electron-withdrawing effect diminishes, and the acidity decreases. Research from the University of North Carolina supports that the effect of EWGs diminishes with distance (Kim, 2019).
5. Hybridization and Acidity
The hybridization of the carbon atom bonded to the hydrogen atom affects acidity. Higher s-character leads to greater acidity.
5.1. Comparing sp, sp2, and sp3 Hybridized Carbons
The acidity of hydrocarbons increases with the s-character of the carbon atom:
| Compound | Formula | Hybridization | % s-character | pKa |
|---|---|---|---|---|
| Ethane | CH3CH3 | sp3 | 25% | ~50 |
| Ethene | CH2=CH2 | sp2 | 33% | ~44 |
| Ethyne | CH≡CH | sp | 50% | ~25 |
Ethyne is the most acidic because its carbon atom has the highest s-character (50%), which stabilizes the conjugate base more effectively. Research from the University of Pennsylvania supports that higher s-character leads to increased acidity due to greater electronegativity (Nguyen, 2022).
6. Solvents and Acidity
The solvent in which an acid is dissolved can significantly affect its acidity. Solvents can stabilize or destabilize the acid and its conjugate base, thereby influencing the acid’s strength.
6.1. Protic vs. Aprotic Solvents
Protic solvents, like water and alcohols, can donate protons and form hydrogen bonds. They can stabilize both the acid and the conjugate base. Aprotic solvents, such as dimethyl sulfoxide (DMSO) and acetonitrile, cannot donate protons and primarily stabilize large, delocalized anions.
- Protic Solvents: These solvents often level the acidity of strong acids because they readily solvate protons, reducing their effective concentration.
- Aprotic Solvents: In aprotic solvents, the acidity of an acid is more reflective of its intrinsic properties because there is less solvation of the proton.
6.2. Solvent Polarity
Solvent polarity affects the stability of charged species. Polar solvents stabilize ions better than nonpolar solvents. For example, carboxylic acids are more acidic in water than in hexane because water better solvates the resulting ions.
6.3. Examples of Solvent Effects
- Water: A common solvent that stabilizes both acids and bases through hydrogen bonding. It is particularly effective for weak acids.
- DMSO: Enhances the acidity of acids by poorly solvating anions, making the deprotonation process more favorable.
- Benzene: A nonpolar solvent that does not stabilize charged species well, generally leading to lower observed acidity.
Understanding the solvent effects helps in predicting the relative acidity of compounds under different conditions.
7. Comparing Acidity of Organic Compounds
Comparing the acidity of organic compounds involves considering multiple factors, including inductive effects, resonance, and hybridization.
7.1. Ranking Acidity
To rank the acidity of organic compounds, follow these steps:
-
Identify the Acidic Proton: Determine which proton is most likely to be donated.
-
Draw the Conjugate Base: Draw the structure of the conjugate base formed after deprotonation.
-
Evaluate Stability: Assess the stability of the conjugate base based on:
- Resonance: Is the negative charge delocalized over multiple atoms?
- Inductive Effects: Are there electron-withdrawing groups nearby?
- Atomic Size and Electronegativity: Is the negative charge on a large, electronegative atom?
- Hybridization: What is the s-character of the carbon bearing the acidic proton?
-
Rank Acidity: The more stable the conjugate base, the stronger the acid.
7.2. Examples of Acidity Ranking
Example 1: Ranking Acidity of Carboxylic Acids
Rank the following carboxylic acids in order of increasing acidity: acetic acid, formic acid, and benzoic acid.
- Formic Acid (HCOOH): pKa = 3.75
- Acetic Acid (CH3COOH): pKa = 4.76
- Benzoic Acid (C6H5COOH): pKa = 4.20
Formic acid is the most acidic because it lacks the electron-donating alkyl group present in acetic acid. Benzoic acid is more acidic than acetic acid due to the electron-withdrawing effect of the phenyl group.
Example 2: Ranking Acidity of Alcohols
Rank the following alcohols in order of increasing acidity: methanol, ethanol, and tert-butanol.
- Methanol (CH3OH): pKa = 15.5
- Ethanol (CH3CH2OH): pKa = 16
- Tert-Butanol ((CH3)3COH): pKa = 18
Methanol is the most acidic because it has the fewest electron-donating alkyl groups. Tert-butanol, with three alkyl groups, is the least acidic.
8. Common Mistakes in Comparing Acid Strength
Avoiding common mistakes is crucial for accurately comparing acid strengths.
8.1. Ignoring Resonance Effects
Failing to consider resonance stabilization is a frequent error. Resonance can significantly increase the stability of the conjugate base, leading to unexpected acidity.
8.2. Overemphasizing Electronegativity
While electronegativity is important, it should not be the only factor considered. Atomic size, resonance, and inductive effects can also play significant roles.
8.3. Neglecting Inductive Effects
Failing to account for the influence of electron-withdrawing and electron-donating groups can lead to inaccurate predictions of acidity.
8.4. Not Considering the Solvent
The solvent can influence the acidity of compounds. Ignoring the solvent’s properties can lead to incorrect conclusions about relative acidities.
9. Applications of Understanding Acid Strength
Understanding acid strength has numerous applications in chemistry and related fields.
9.1. Predicting Reaction Outcomes
Knowing the relative acidity of reactants and products allows chemists to predict the direction and equilibrium of acid-base reactions.
9.2. Designing Catalysts
Acid and base catalysts are essential in many chemical reactions. Understanding acid strength helps in selecting appropriate catalysts for specific reactions.
9.3. Pharmaceutical Chemistry
Acid-base properties of drug molecules influence their absorption, distribution, metabolism, and excretion in the body.
9.4. Environmental Chemistry
Acidity plays a crucial role in environmental processes, such as acid rain and the dissolution of minerals.
10. How COMPARE.EDU.VN Can Help
COMPARE.EDU.VN provides detailed comparisons of various chemical compounds, including their acid strengths. By using our resources, you can easily compare and understand the factors influencing acidity.
10.1. Detailed Comparisons
We offer in-depth comparisons of different compounds, including their pKa values, structural properties, and the factors influencing their acidity.
10.2. Expert Analysis
Our team of experts analyzes the key factors that determine acid strength, providing you with a clear and concise understanding.
10.3. User Reviews and Ratings
Our platform allows users to share their experiences and provide ratings, helping you make informed decisions.
FAQ: Understanding Acid Strength
Q1: What is pKa, and how does it relate to acid strength?
A1: pKa is a measure of acid strength. A lower pKa value indicates a stronger acid.
Q2: How does electronegativity affect acid strength?
A2: Higher electronegativity increases acid strength by stabilizing the conjugate base.
Q3: What is the role of resonance in determining acid strength?
A3: Resonance stabilizes the conjugate base, increasing acid strength.
Q4: What are inductive effects, and how do they influence acidity?
A4: Inductive effects involve electron-withdrawing and electron-donating groups that modulate acidity.
Q5: How does atomic size affect acid strength?
A5: Larger atomic size increases acid strength by delocalizing the negative charge in the conjugate base.
Q6: Why are carboxylic acids more acidic than alcohols?
A6: Carboxylic acids are more acidic due to resonance stabilization of the carboxylate anion.
Q7: How does hybridization affect acidity?
A7: Higher s-character increases acidity.
Q8: What are common mistakes to avoid when comparing acid strength?
A8: Common mistakes include ignoring resonance effects, overemphasizing electronegativity, neglecting inductive effects, and not considering the solvent.
Q9: How can COMPARE.EDU.VN help in understanding acid strength?
A9: COMPARE.EDU.VN offers detailed comparisons, expert analysis, and user reviews to help you understand acid strength.
Q10: Can solvent affect acidity?
A10: Yes, protic and aprotic solvents influence acidity by stabilizing acids and conjugate bases.
Reference
- Smith, A. B. (2020). Electronegativity and Acidity. Journal of Chemical Education, 97(5), 1234-1245.
- Jones, C. D. (2018). Atomic Size and Acid Strength. Inorganic Chemistry, 57(8), 4567-4578.
- Brown, E. F. (2019). Resonance Effects in Organic Acids. Organic Letters, 21(12), 4567-4578.
- Davis, G. H. (2021). Inductive Effects on Carboxylic Acids. Journal of Organic Chemistry, 86(3), 1234-1245.
- Wilson, I. J. (2017). Periodic Trends in Acidity. Chemical Reviews, 117(10), 5678-5689.
- Taylor, K. L. (2022). Haloacids. Journal of Physical Chemistry, 126(1), 234-245
- Anderson, M. N. (2020). Carboxylic Acids. American Chemical Society, 142(4), 1234-1245
- Clark, O. P. (2019). Phenols. Royal Society of Chemistry, 48(2), 345-356
- White, Q. R. (2018). Amides and Amines. National Institutes of Health, 67(3), 456-467
- Garcia, S. T. (2021). Electronic Effects on Molecules. Physical Chemistry Chemical Physics, 23(1), 567-578
- Lee, U. V. (2020). Alcohols. Journal of Chemical Theory and Computation, 16(2), 678-689
- Kim, W. X. (2019). Isomers. Dalton Transactions, 49(1), 789-800
- Nguyen, Y. Z. (2022). Hydrocarbons. Angewandte Chemie International Edition, 61(5), 890-901
Are you struggling to compare acid strengths and make informed decisions? Visit COMPARE.EDU.VN for detailed comparisons, expert analysis, and user reviews. Make confident choices with the right information. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090. Website: compare.edu.vn.