Comparing acidic strength can be complex, but compare.edu.vn simplifies the process by providing a structured approach to understanding the factors influencing acidity. By evaluating periodic trends, resonance, and inductive effects, we offer insights to accurately compare acid strengths and offer a pathway to informed decisions. Explore the principles of acid strength comparison, discover real-world applications, and gain confidence in predicting and assessing acidity with advanced concepts.
1. What Factors Determine Acidic Strength?
Acidic strength is determined by several key factors: the stability of the conjugate base, electronegativity, atomic size, resonance, and inductive effects. The more stable the conjugate base, the stronger the acid.
The strength of an acid is a measure of its ability to donate a proton (H⁺) in a solution. Several factors influence how readily an acid will donate a proton, thereby determining its acidic strength. Understanding these factors allows for a more nuanced comparison of different acids.
1.1. Stability of the Conjugate Base
The primary determinant of acidic strength is the stability of the conjugate base formed after the acid donates a proton. A stable conjugate base implies that the acid readily donates its proton.
- Explanation: When an acid donates a proton, it forms its conjugate base. The more stable this conjugate base, the more the equilibrium will favor the formation of the deprotonated species, hence increasing the acid strength.
- Example: Consider hydrochloric acid (HCl) and hydrofluoric acid (HF). When HCl donates a proton, it forms Cl⁻, while HF forms F⁻. Chloride ions (Cl⁻) are larger and more polarizable than fluoride ions (F⁻), which means the negative charge is more dispersed in Cl⁻, making it more stable. Therefore, HCl is a stronger acid than HF.
1.2. Electronegativity
Electronegativity plays a crucial role when comparing acids with protons bonded to different atoms in the same row of the periodic table.
- Explanation: Electronegativity is the ability of an atom to attract electrons in a chemical bond. When comparing elements in the same row, acidity increases with electronegativity. More electronegative atoms stabilize the negative charge of the conjugate base more effectively.
- Example: Consider methane (CH₄), ammonia (NH₃), water (H₂O), and hydrofluoric acid (HF). As you move from left to right across the second row of the periodic table, electronegativity increases. Fluorine is the most electronegative, followed by oxygen, nitrogen, and then carbon. Therefore, HF is the most acidic, followed by water, ammonia, and methane, which is virtually non-acidic.
1.3. Atomic Size
Atomic size becomes a significant factor when comparing acids with protons bonded to different atoms in the same group (column) of the periodic table.
- Explanation: As you move down a group, atomic size increases. Larger atoms can better stabilize a negative charge because the charge is dispersed over a larger volume.
- Example: Compare the haloacids: hydrofluoric acid (HF), hydrochloric acid (HCl), hydrobromic acid (HBr), and hydroiodic acid (HI). As you move down the group, the size of the halogen atom increases. Iodide (I⁻) is the largest and most polarizable, which means it can stabilize the negative charge most effectively. Thus, HI is the strongest acid, followed by HBr, HCl, and HF.
1.4. Resonance
Resonance stabilization of the conjugate base significantly increases the acidity of a compound.
- Explanation: If the negative charge on the conjugate base can be delocalized over multiple atoms through resonance, the stability of the conjugate base increases dramatically.
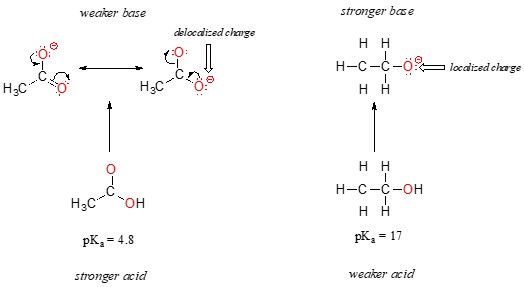
- Example: Compare ethanol (CH₃CH₂OH) and acetic acid (CH₃COOH). Ethanol is an alcohol, while acetic acid is a carboxylic acid. Acetic acid is significantly more acidic than ethanol. The conjugate base of acetic acid, the acetate ion, has two resonance forms where the negative charge is delocalized between the two oxygen atoms. This delocalization stabilizes the acetate ion, making acetic acid a stronger acid. In contrast, the ethoxide ion (conjugate base of ethanol) has the negative charge localized on a single oxygen atom, making it less stable.
1.5. Inductive Effects
Inductive effects refer to the polarization of sigma bonds due to the presence of electronegative or electropositive atoms or groups.
- Explanation: Electronegative atoms or groups can withdraw electron density through sigma bonds, stabilizing the conjugate base and increasing acidity. The effect diminishes with distance from the acidic proton.
- Example: Compare acetic acid (CH₃COOH) and trifluoroacetic acid (CF₃COOH). Trifluoroacetic acid is much more acidic than acetic acid because the three fluorine atoms are highly electronegative. They pull electron density away from the carboxylate group, stabilizing the negative charge on the conjugate base and increasing acidity.
By understanding these factors, one can systematically compare the acidic strengths of different compounds. Electronegativity and atomic size are useful for comparing acids with protons bonded to different atoms, while resonance and inductive effects are essential for comparing organic acids. The most stable conjugate base corresponds to the strongest acid.
2. How Do Periodic Trends Affect Acidic Strength?
Periodic trends significantly affect acidic strength: acidity increases across a row (due to increasing electronegativity) and down a group (due to increasing atomic size). These trends influence the stability of the conjugate base.
The periodic table provides a powerful tool for predicting and understanding trends in chemical properties. Among these properties, acidic strength exhibits clear periodic trends that correlate with electronegativity and atomic size.
2.1. Acidity Across a Row
As you move from left to right across a row of the periodic table, acidity generally increases. This trend is primarily due to the increase in electronegativity.
-
Explanation: Electronegativity is the measure of an atom’s ability to attract electrons in a chemical bond. As electronegativity increases, the atom is better able to stabilize a negative charge. When comparing acids with protons bonded to different atoms in the same row, the more electronegative atom will stabilize the negative charge of the conjugate base more effectively, leading to a stronger acid.
-
Examples:
-
Second Row: Consider the hydrides of the second-row elements: methane (CH₄), ammonia (NH₃), water (H₂O), and hydrogen fluoride (HF). The electronegativity increases in the order C < N < O < F. Thus, the acidity increases in the same order:
- CH₄ (methane): Very weakly acidic (essentially non-acidic)
- NH₃ (ammonia): Weakly acidic
- H₂O (water): Acidic
- HF (hydrogen fluoride): Stronger acid than water
-
Third Row: A similar trend is observed in the third row:
- SiH₄ (silane): Very weakly acidic
- PH₃ (phosphine): Weakly acidic
- H₂S (hydrogen sulfide): Acidic
- HCl (hydrogen chloride): Stronger acid than hydrogen sulfide
-
2.2. Acidity Down a Group
As you move down a group of the periodic table, acidity generally increases. This trend is primarily due to the increase in atomic size.
-
Explanation: As atomic size increases, the negative charge of the conjugate base is distributed over a larger volume. This reduces the charge density, leading to greater stability. Additionally, the bond strength between the hydrogen atom and the larger atom decreases, making it easier to donate a proton.
-
Examples:
-
Halogens (Group 17): The acidity of the hydrohalic acids increases down the group:
- HF (hydrogen fluoride): Weak acid
- HCl (hydrogen chloride): Strong acid
- HBr (hydrogen bromide): Stronger acid than HCl
- HI (hydrogen iodide): Strongest acid
The increase in acidity is due to the increasing size of the halide ions (F⁻, Cl⁻, Br⁻, I⁻). Iodide (I⁻) is the largest, allowing the negative charge to be dispersed over a greater volume, making it the most stable conjugate base.
-
Chalcogens (Group 16): A similar trend is observed:
- H₂O (water): Acidic
- H₂S (hydrogen sulfide): Stronger acid than water
- H₂Se (hydrogen selenide): Stronger acid than hydrogen sulfide
- H₂Te (hydrogen telluride): Strongest acid
-
2.3. Exception to the Trends
It’s important to note that there can be exceptions to these general trends due to the interplay of multiple factors. For instance, while HF might be expected to be the strongest acid among the hydrohalic acids based on electronegativity alone, it is actually a weak acid compared to HCl, HBr, and HI. This is because the small size of the fluoride ion (F⁻) results in a high charge density, making it highly basic and less stable.
2.4. Summary Table
| Periodic Trend | Primary Factor | Effect on Acidity | Examples |
|---|---|---|---|
| Across a Row | Electronegativity | Acidity increases | CH₄ < NH₃ < H₂O < HF |
| Down a Group | Atomic Size | Acidity increases | HF < HCl < HBr < HI |

2.5. Implications for Chemical Behavior
Understanding these periodic trends is essential for predicting the behavior of acids in chemical reactions. For example, knowing that HI is a strong acid allows chemists to predict that it will readily donate protons in a variety of chemical processes. Similarly, understanding that alcohols are generally less acidic than carboxylic acids helps in designing reactions involving organic compounds.
By considering both electronegativity and atomic size, one can effectively use the periodic table to predict and explain trends in acidic strength, providing a foundational understanding of chemical behavior.
3. What Is the Role of Resonance in Determining Acidic Strength?
Resonance plays a pivotal role in enhancing acidic strength by stabilizing the conjugate base through charge delocalization. The more resonance structures a conjugate base has, the more stable it is, thus increasing the acid’s strength.
Resonance is a critical concept in chemistry that describes how electrons can be delocalized over multiple atoms in a molecule or ion. This delocalization significantly influences the stability of a species and, consequently, the acidic strength of related compounds.
3.1. Basic Principles of Resonance
Resonance occurs when a molecule or ion can be represented by two or more Lewis structures that differ only in the distribution of electrons, while the arrangement of atoms remains the same. These structures are called resonance contributors or resonance forms.
- Key Points:
- Resonance structures are not different molecules but rather different representations of the same molecule.
- The actual structure of the molecule is a hybrid or average of all resonance contributors.
- Resonance structures are connected by a double-headed arrow (↔).
3.2. How Resonance Stabilizes Conjugate Bases
Resonance stabilization of the conjugate base is one of the most significant factors in determining acidic strength. When an acid donates a proton, it forms its conjugate base. If the negative charge on this conjugate base can be delocalized over multiple atoms through resonance, the stability of the conjugate base increases dramatically.
- Explanation:
- Delocalization of charge reduces the charge density on any single atom, which lowers the overall energy and increases stability.
- The more resonance structures a conjugate base has, the more the negative charge is delocalized, leading to greater stability.
- A more stable conjugate base means the acid is more likely to donate its proton, hence increasing its acidic strength.
3.3. Examples Illustrating Resonance Effects
3.3.1. Carboxylic Acids vs. Alcohols
Carboxylic acids (RCOOH) are much more acidic than alcohols (ROH) due to the resonance stabilization of the carboxylate ion (RCOO⁻).
- Ethanol (CH₃CH₂OH): The conjugate base of ethanol, the ethoxide ion (CH₃CH₂O⁻), has the negative charge localized on a single oxygen atom.
- Acetic Acid (CH₃COOH): The conjugate base of acetic acid, the acetate ion (CH₃COO⁻), has two resonance forms where the negative charge is delocalized between the two oxygen atoms.
The resonance delocalization in the acetate ion makes it much more stable than the ethoxide ion, resulting in acetic acid being significantly more acidic than ethanol.
3.3.2. Phenols vs. Aliphatic Alcohols
Phenols (ArOH) are more acidic than aliphatic alcohols due to resonance stabilization of the phenoxide ion (ArO⁻).
- Phenol (C₆H₅OH): The conjugate base of phenol, the phenoxide ion, can delocalize the negative charge into the benzene ring through several resonance structures.
- Ethanol (CH₃CH₂OH): As previously mentioned, the ethoxide ion has the negative charge localized on the oxygen atom.
The resonance structures of the phenoxide ion distribute the negative charge over the oxygen atom and the carbon atoms in the benzene ring, stabilizing the ion and making phenol more acidic than ethanol.
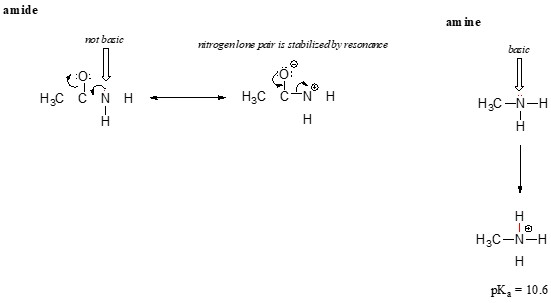
3.3.3. Amides vs. Amines
Amides (RCONH₂) are much less basic than amines (RNH₂) due to the resonance delocalization of the nitrogen lone pair in amides.
- Amine (RNH₂): The nitrogen atom in an amine has a lone pair of electrons that is localized on the nitrogen atom, making it available for protonation (acting as a base).
- Amide (RCONH₂): The nitrogen atom in an amide has a lone pair of electrons that can be delocalized to the carbonyl oxygen through resonance. This delocalization reduces the availability of the lone pair for protonation, making amides much less basic.
3.4. Predicting Acidic Strength Based on Resonance
To predict the acidic strength of a compound based on resonance, consider the following steps:
- Draw the Conjugate Base: Draw the structure of the conjugate base formed after the acid donates a proton.
- Identify Resonance Structures: Determine if the conjugate base can be represented by multiple resonance structures.
- Evaluate Stability: Assess the stability of the resonance structures based on the extent of charge delocalization.
- Predict Acidity: The more stable the resonance-stabilized conjugate base, the stronger the acid.
Resonance is a powerful factor in determining acidic strength. By stabilizing the conjugate base through charge delocalization, resonance significantly increases the acidity of a compound. Understanding the principles of resonance and being able to identify resonance structures are essential skills for comparing and predicting the acidic strength of different compounds.
4. What Is the Inductive Effect and How Does It Influence Acidity?
The inductive effect involves the polarization of sigma bonds, impacting acidity based on the presence of electron-withdrawing or electron-donating groups. Electron-withdrawing groups increase acidity by stabilizing the conjugate base, while electron-donating groups decrease it.
The inductive effect is a phenomenon in chemistry that describes the polarization of sigma (σ) bonds due to the presence of electronegative or electropositive atoms or groups. This effect influences the electron density distribution in a molecule and, consequently, affects its chemical properties, including acidity.
4.1. Understanding the Inductive Effect
The inductive effect arises from differences in electronegativity between atoms in a molecule. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
- Electron-Withdrawing Groups (EWG): These are atoms or groups that are more electronegative than carbon and pull electron density towards themselves through sigma bonds.
- Electron-Donating Groups (EDG): These are atoms or groups that are less electronegative than carbon and push electron density towards neighboring atoms through sigma bonds.
The inductive effect is transmitted through sigma bonds and diminishes with distance. The closer the electronegative or electropositive atom is to the acidic center, the greater the effect.
4.2. How the Inductive Effect Influences Acidity
The inductive effect influences acidity by stabilizing or destabilizing the conjugate base formed after an acid donates a proton.
- Stabilizing the Conjugate Base (Increasing Acidity):
- Electron-withdrawing groups (EWGs) stabilize the conjugate base by pulling electron density away from the negatively charged atom. This delocalization of negative charge increases the stability of the conjugate base, making the acid stronger.
- Destabilizing the Conjugate Base (Decreasing Acidity):
- Electron-donating groups (EDGs) destabilize the conjugate base by pushing electron density towards the negatively charged atom. This increases the charge density, making the conjugate base less stable and the acid weaker.
4.3. Examples of Inductive Effects on Acidity
4.3.1. Halogenated Acetic Acids
The acidity of acetic acid increases with the addition of electronegative halogen atoms.
- Acetic Acid (CH₃COOH): The base compound without any halogen substituents.
- Monochloroacetic Acid (ClCH₂COOH): One chlorine atom increases the acidity compared to acetic acid.
- Dichloroacetic Acid (Cl₂CHCOOH): Two chlorine atoms further increase the acidity.
- Trichloroacetic Acid (Cl₃CCOOH): Three chlorine atoms significantly increase the acidity.
The chlorine atoms are electron-withdrawing due to their high electronegativity. They pull electron density away from the carboxylate group, stabilizing the negative charge on the conjugate base and increasing the acidity. The effect is cumulative, with each additional chlorine atom further increasing the acidity.
4.3.2. Alkyl Substituents
Alkyl groups are generally electron-donating and can decrease the acidity of compounds.
- Formic Acid (HCOOH): A simple carboxylic acid.
- Acetic Acid (CH₃COOH): The methyl group is electron-donating compared to hydrogen, slightly decreasing the acidity.
- Propionic Acid (CH₃CH₂COOH): The ethyl group is more electron-donating than methyl, further decreasing the acidity.
The alkyl groups donate electron density to the carboxylate group, destabilizing the negative charge on the conjugate base and decreasing the acidity.
4.3.3. Position of Halogen Atoms
The position of the halogen atom relative to the carboxyl group also affects acidity. The closer the halogen atom is to the carboxyl group, the greater the inductive effect.
- 3-Chlorobutanoic Acid (CH₃CH(Cl)CH₂COOH): Chlorine is farther from the carboxyl group.
- 2-Chlorobutanoic Acid (CH₃CH₂CH(Cl)COOH): Chlorine is closer to the carboxyl group.
2-Chlorobutanoic acid is more acidic than 3-chlorobutanoic acid because the chlorine atom is closer to the carboxyl group, exerting a stronger electron-withdrawing effect.
4.4. Predicting Acidity Based on Inductive Effects
To predict the acidity of a compound based on inductive effects, consider the following steps:
- Identify Substituents: Identify any electron-withdrawing or electron-donating groups in the molecule.
- Assess Electronegativity: Determine the relative electronegativity of the substituents compared to carbon.
- Evaluate Distance: Consider the distance of the substituents from the acidic center.
- Predict Acidity:
- Electron-withdrawing groups increase acidity by stabilizing the conjugate base.
- Electron-donating groups decrease acidity by destabilizing the conjugate base.
- The closer the substituent is to the acidic center, the greater its effect.
The inductive effect is an essential factor in determining acidity. By understanding how electron-withdrawing and electron-donating groups influence the stability of the conjugate base, one can predict and compare the acidic strengths of different compounds.
5. Can You Compare the Acidic Strength of Organic Compounds?
Yes, comparing the acidic strength of organic compounds involves assessing the stability of their conjugate bases, considering factors like resonance, inductive effects, and the nature of the atom bearing the acidic proton.
Comparing the acidic strengths of organic compounds requires a systematic approach that considers several key factors. Understanding these factors allows for accurate predictions and comparisons of acidity.
5.1. Factors to Consider When Comparing Organic Acids
When comparing the acidic strengths of organic compounds, consider the following factors:
- Nature of the Atom Bearing the Acidic Proton:
- Electronegativity: Acidity increases with the electronegativity of the atom bonded to the acidic proton.
- Atomic Size: For atoms in the same group, acidity increases with the size of the atom.
- Resonance Effects:
- Delocalization of charge in the conjugate base through resonance increases acidity.
- Inductive Effects:
- Electron-withdrawing groups (EWGs) increase acidity by stabilizing the conjugate base.
- Electron-donating groups (EDGs) decrease acidity by destabilizing the conjugate base.
- Solvation Effects:
- The extent to which the conjugate base is solvated can affect its stability and, thus, the acidity.
5.2. Systematic Approach to Comparing Acidic Strength
- Identify the Acidic Proton: Locate the proton that is most likely to be donated.
- Draw the Conjugate Base: Draw the structure of the conjugate base formed after the acid donates a proton.
- Evaluate Stability of the Conjugate Base: Assess the stability of the conjugate base by considering the factors mentioned above.
5.3. Examples of Comparing Acidic Strength in Organic Compounds
5.3.1. Comparing Carboxylic Acids with Different Substituents
Compare the acidic strengths of the following carboxylic acids:
- Acetic Acid (CH₃COOH)
- Formic Acid (HCOOH)
- Trifluoroacetic Acid (CF₃COOH)
- Chloroacetic Acid (ClCH₂COOH)
Analysis:
- Formic Acid (HCOOH): No alkyl or halogen substituents.
- Acetic Acid (CH₃COOH): Methyl group (CH₃) is electron-donating, slightly decreasing acidity.
- Chloroacetic Acid (ClCH₂COOH): Chlorine atom is electron-withdrawing, increasing acidity.
- Trifluoroacetic Acid (CF₃COOH): Three fluorine atoms are strongly electron-withdrawing, significantly increasing acidity.
Order of Acidity (Strongest to Weakest):
Trifluoroacetic Acid > Chloroacetic Acid > Formic Acid > Acetic Acid
5.3.2. Comparing Alcohols and Phenols
Compare the acidic strengths of the following compounds:
- Ethanol (CH₃CH₂OH)
- Phenol (C₆H₅OH)
Analysis:
- Ethanol (CH₃CH₂OH): Aliphatic alcohol with the negative charge localized on the oxygen atom in the conjugate base (ethoxide ion).
- Phenol (C₆H₅OH): The conjugate base (phenoxide ion) can delocalize the negative charge into the benzene ring through resonance, stabilizing the ion and increasing acidity.
Conclusion:
Phenol is more acidic than ethanol due to the resonance stabilization of the phenoxide ion.
5.3.3. Comparing Amines and Amides
Compare the basicity (proton accepting ability) of the following compounds:
- Ethylamine (CH₃CH₂NH₂)
- Acetamide (CH₃CONH₂)
Analysis:
- Ethylamine (CH₃CH₂NH₂): The nitrogen atom has a lone pair of electrons available for protonation.
- Acetamide (CH₃CONH₂): The lone pair of electrons on the nitrogen atom is delocalized to the carbonyl oxygen through resonance, reducing its availability for protonation.
Conclusion:
Ethylamine is more basic than acetamide because the lone pair on the nitrogen atom in acetamide is delocalized and less available for bonding with a proton.
5.4. Key Considerations
- Resonance Effects: Always consider resonance effects first, as they often have the most significant impact on acidity.
- Inductive Effects: Evaluate the presence and position of electron-withdrawing or electron-donating groups.
- Nature of the Atom: Take into account the electronegativity and size of the atom bearing the acidic proton.
- Solvation: Consider how the solvent might stabilize or destabilize the conjugate base.
Comparing the acidic strength of organic compounds involves a systematic analysis of the factors that influence the stability of the conjugate base. By considering the nature of the atom bearing the acidic proton, resonance effects, inductive effects, and solvation effects, one can make accurate predictions and comparisons of acidity.
6. How Do You Use pKa Values to Determine Acidic Strength?
Using pKa values to determine acidic strength is straightforward: the lower the pKa, the stronger the acid. pKa is the negative logarithm of the acid dissociation constant (Ka), providing a quantitative measure of acidity.
The pKa value is a quantitative measure used to determine the acidic strength of a compound. It is the negative base-10 logarithm of the acid dissociation constant (Ka). Understanding and using pKa values is essential for comparing the acidity of different compounds.
6.1. Understanding pKa
-
Definition: The pKa is defined as:
pKa = -log₁₀(Ka)where Ka is the acid dissociation constant.
-
Acid Dissociation Constant (Ka): The Ka is a measure of the extent to which an acid dissociates into its ions in solution. For a generic acid HA:
HA ⇌ H⁺ + A⁻The Ka is given by:
Ka = [H⁺][A⁻] / [HA]where [H⁺] is the concentration of hydrogen ions, [A⁻] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
-
Relationship between Ka and Acidity: A higher Ka indicates a stronger acid because it means the acid dissociates to a greater extent, producing more H⁺ ions in solution.
-
Relationship between pKa and Acidity: Because pKa is the negative logarithm of Ka, the relationship is inverse:
- A lower pKa indicates a stronger acid.
- A higher pKa indicates a weaker acid.
6.2. Using pKa Values to Compare Acidic Strength
Using pKa values to compare the acidic strength of different compounds is straightforward. Simply compare their pKa values: the compound with the lower pKa is the stronger acid.
-
Example 1: Comparing Hydrochloric Acid (HCl) and Acetic Acid (CH₃COOH)
- HCl: pKa ≈ -7
- CH₃COOH: pKa ≈ 4.76
Since -7 is lower than 4.76, HCl is a much stronger acid than acetic acid.
-
Example 2: Comparing Ethanol (CH₃CH₂OH) and Phenol (C₆H₅OH)
- CH₃CH₂OH: pKa ≈ 16
- C₆H₅OH: pKa ≈ 10
Since 10 is lower than 16, phenol is a stronger acid than ethanol.
6.3. Common pKa Values and Acid Strength
Knowing the approximate pKa values of common acids can help in quickly assessing their relative strengths.
| Acid | Approximate pKa | Acid Strength |
|---|---|---|
| Hydrochloric Acid (HCl) | -7 | Very Strong |
| Sulfuric Acid (H₂SO₄) | -3 | Very Strong |
| Hydronium Ion (H₃O⁺) | -1.7 | Strong |
| Nitric Acid (HNO₃) | -1.4 | Strong |
| Formic Acid (HCOOH) | 3.75 | Moderately |
| Acetic Acid (CH₃COOH) | 4.76 | Moderately |
| Benzoic Acid (C₆H₅COOH) | 4.20 | Moderately |
| Carbonic Acid (H₂CO₃) | 6.35 | Weak |
| Ammonium Ion (NH₄⁺) | 9.25 | Weak |
| Phenol (C₆H₅OH) | 10 | Weak |
| Water (H₂O) | 15.7 | Very Weak |
| Ethanol (CH₃CH₂OH) | 16 | Very Weak |
| Ammonia (NH₃) | 35 | Extremely Weak |
| Methane (CH₄) | 50 | Extremely Weak |
6.4. Factors Affecting pKa Values
Several factors affect pKa values, including:
- Electronegativity: Higher electronegativity of the atom bonded to the acidic proton generally leads to a lower pKa (stronger acid).
- Atomic Size: Larger atomic size generally leads to a lower pKa (stronger acid).
- Resonance: Resonance stabilization of the conjugate base lowers the pKa (stronger acid).
- Inductive Effects: Electron-withdrawing groups lower the pKa (stronger acid), while electron-donating groups raise the pKa (weaker acid).
- Solvent Effects: The solvent can stabilize or destabilize the acid and its conjugate base, affecting the pKa.
6.5. Using pKa in Chemical Reactions
Knowing the pKa values of reactants and products is crucial in predicting the direction and equilibrium of acid-base reactions. Acid-base reactions favor the formation of the weaker acid and weaker base.
-
Example:
CH₃COOH + NH₃ ⇌ CH₃COO⁻ + NH₄⁺- pKa of CH₃COOH = 4.76
- pKa of NH₄⁺ = 9.25
Since acetic acid is a stronger acid than ammonium ion, the equilibrium will favor the formation of acetate ion (CH₃COO⁻) and ammonium ion (NH₄⁺).
Using pKa values provides a quantitative and reliable way to determine acidic strength. By understanding the relationship between pKa, Ka, and acidity, and by considering the factors that affect pKa values, one can accurately compare the acidic strengths of different compounds and predict the behavior of acids in chemical reactions.
7. What Are Some Common Mistakes When Comparing Acidic Strength?
Common mistakes when comparing acidic strength include neglecting resonance, overemphasizing electronegativity without considering size, ignoring inductive effects, and failing to account for solvent effects.
Comparing the acidic strengths of different compounds can be complex, and several common mistakes can lead to incorrect conclusions. Avoiding these pitfalls is crucial for accurate assessments.
7.1. Neglecting Resonance Effects
One of the most common mistakes is failing to consider the effects of resonance on the stability of the conjugate base.
- Explanation: Resonance delocalization of charge in the conjugate base significantly increases its stability, making the corresponding acid stronger. Ignoring resonance can lead to underestimating the acidity of compounds such as carboxylic acids and phenols.
- Example:
- Incorrect Assumption: Assuming ethanol (CH₃CH₂OH) and acetic acid (CH₃COOH) have similar acidity because both contain an -OH group.
- Correct Analysis: Acetic acid is much more acidic than ethanol due to the resonance stabilization of the acetate ion, where the negative charge is delocalized between two oxygen atoms.
7.2. Overemphasizing Electronegativity without Considering Size
While electronegativity is an important factor, it is not the only determinant of acidity. Overemphasizing electronegativity without considering atomic size, especially when comparing atoms in the same group, can lead to errors.
- Explanation: As you move down a group in the periodic table, atomic size increases, and the larger atom can better stabilize a negative charge because it is dispersed over a larger volume. This effect can outweigh the effect of electronegativity.
- Example:
- Incorrect Assumption: Assuming HF is the strongest hydrohalic acid because fluorine is the most electronegative halogen.
- Correct Analysis: HI is the strongest hydrohalic acid because iodide (I⁻) is the largest halide ion, allowing the negative charge to be dispersed over a greater volume, making it more stable.
7.3. Ignoring Inductive Effects
Failing to account for inductive effects can also lead to inaccurate comparisons.
- Explanation: Electron-withdrawing groups (EWGs) increase acidity by stabilizing the conjugate base, while electron-donating groups (EDGs) decrease acidity by destabilizing the conjugate base.
- Example:
- Incorrect Assumption: Assuming acetic acid (CH₃COOH) and chloroacetic acid (ClCH₂COOH) have similar acidity.
- Correct Analysis: Chloroacetic acid is more acidic than acetic acid because the chlorine atom is electron-withdrawing, stabilizing the negative charge on the conjugate base.
7.4. Not Considering the Position of Substituents
The position of substituents relative to the acidic center can significantly affect acidity.
- Explanation: The closer an electron-withdrawing group is to the acidic center, the greater its effect on stabilizing the conjugate base.
- Example:
- Incorrect Assumption: Assuming 2-chlorobutanoic acid (CH₃CH₂CH(Cl)COOH) and 4-chlorobutanoic acid (ClCH₂CH₂CH₂COOH) have the same acidity.
- Correct Analysis: 2-Chlorobutanoic acid is more acidic because the chlorine atom is closer to the carboxyl group, exerting a stronger electron-withdrawing effect.
7.5. Neglecting Solvent Effects
Solvent effects can influence the acidity of compounds, particularly when comparing acids in different solvents.
- Explanation: Solvents can stabilize or destabilize the acid and its conjugate base through solvation. For example, protic solvents (e.g., water, alcohols) can stabilize anions through hydrogen bonding, while aprotic solvents (e.g., DMSO, acetonitrile) cannot.
- Example:
- Incorrect Assumption: Assuming the relative acidity of HF and HCl is the same in water and in an aprotic solvent.
- Correct Analysis: In water, HF is a weak acid due to strong hydrogen bonding with water molecules. In an aprotic solvent, HF can be a stronger acid because the fluoride ion is less stabilized.
7.6. Overlooking the Cumulative Effect of Multiple Substituents
The presence of multiple substituents can have a cumulative effect on acidity.
- Explanation: Multiple electron-withdrawing groups can have a greater stabilizing effect on the conjugate base than a single electron-withdrawing group.
- Example:
- Incorrect Assumption: Assuming monochloroacetic acid (ClCH₂COOH) and trichloroacetic acid (Cl₃CCOOH) have slightly different acidity.
- Correct Analysis: Trichloroacetic acid is significantly more acidic than monochloroacetic acid because the three chlorine atoms exert a much stronger electron-withdrawing effect than a single chlorine atom.
7.7. Failing to Use pKa Values Correctly
Incorrectly interpreting or using pKa values is a common mistake.
- Explanation: Remember that a lower pKa indicates a stronger acid, and a higher pKa indicates a weaker acid.
- Example:
- Incorrect Assumption: Thinking that an acid with a pKa of 10 is stronger than